

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203332 (CHEMBL3913908) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274157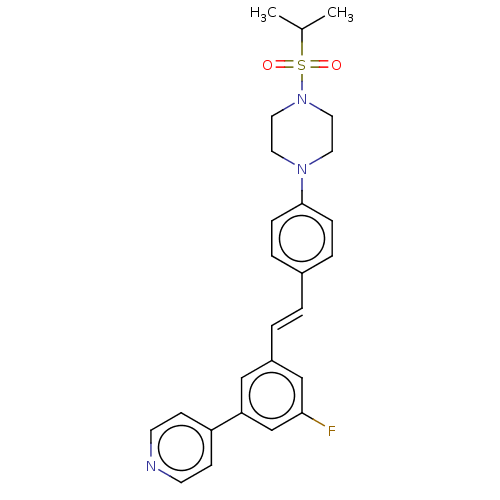 (CHEMBL4127656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274165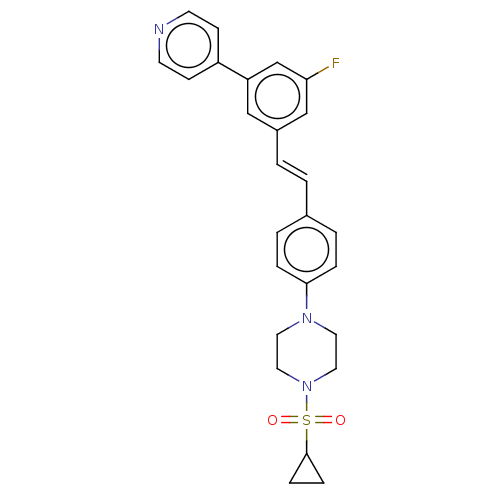 (CHEMBL4126996) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274156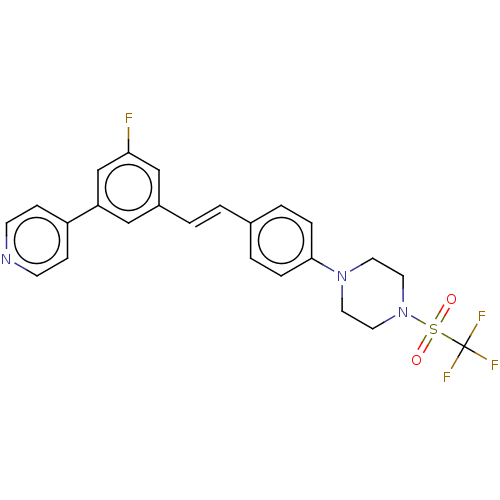 (CHEMBL4128368) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203339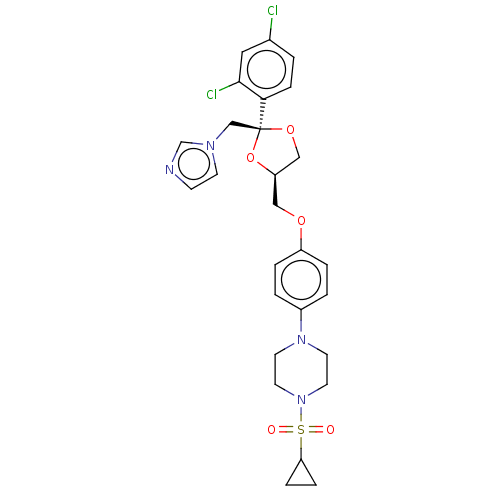 (CHEMBL3922888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274164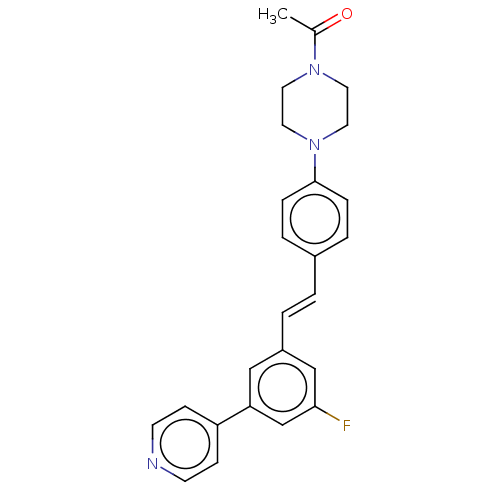 (CHEMBL4129721) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330386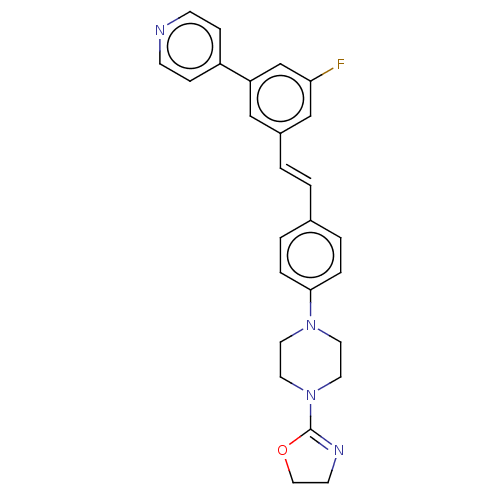 (US9725436, Entry 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274146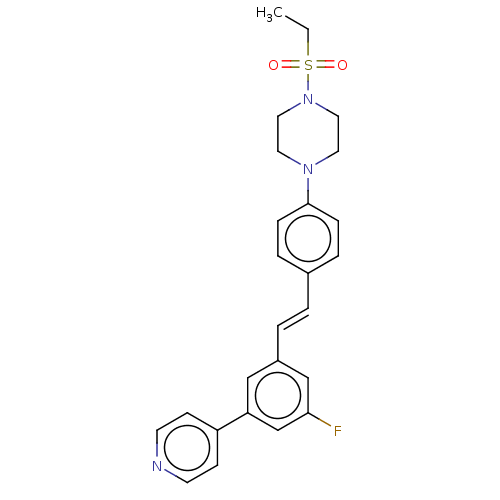 (CHEMBL4126284) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330388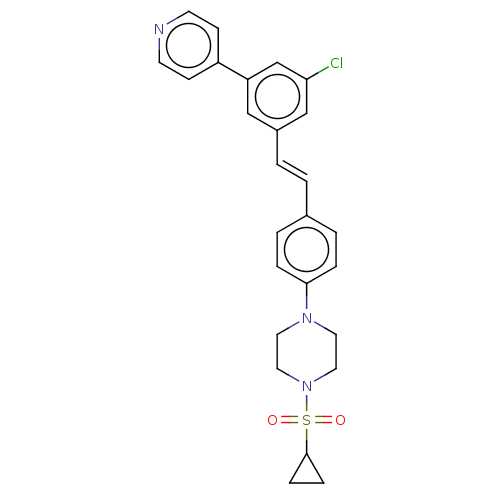 (US9725436, Entry 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330391 (US9725436, Entry 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330383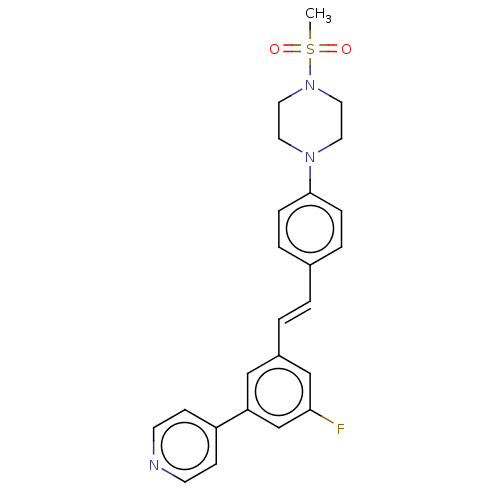 (US9725436, Entry 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274147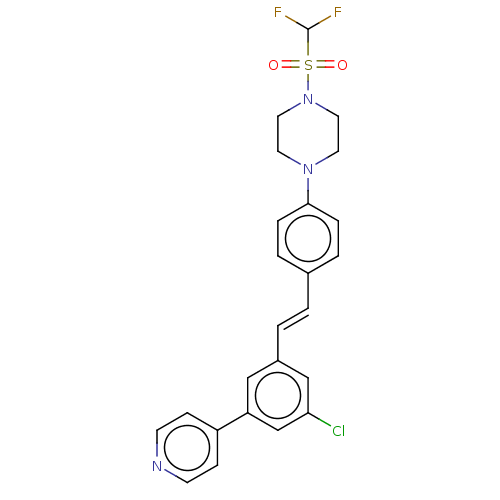 (CHEMBL4129859) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274166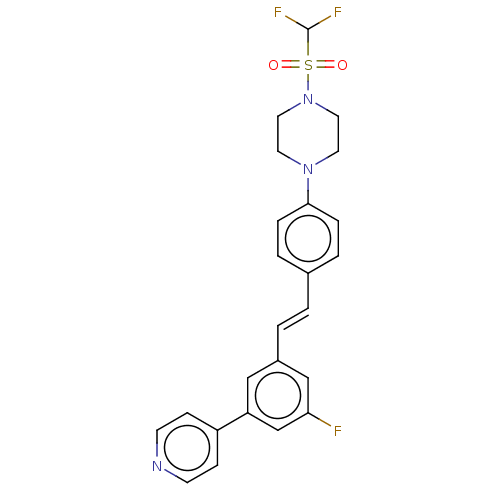 (CHEMBL4126956) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274155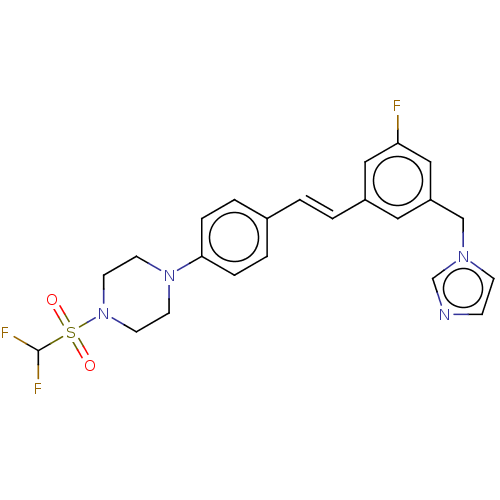 (CHEMBL4127131) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330384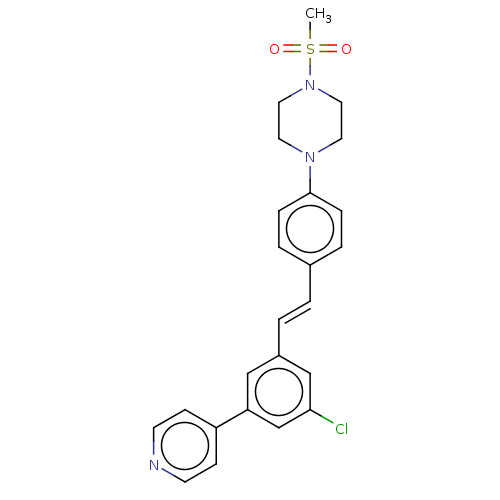 (US9725436, Entry 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330389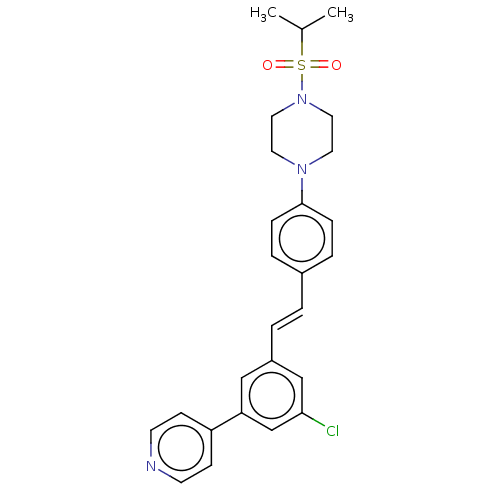 (US9725436, Entry 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274162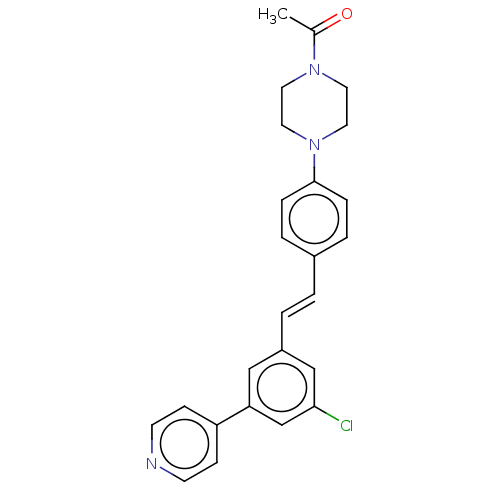 (CHEMBL4127399) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274163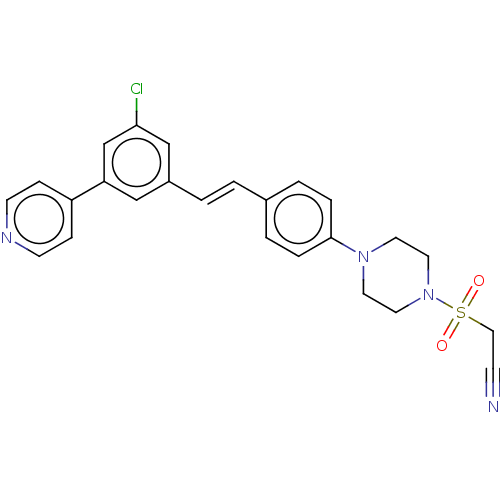 (CHEMBL4128495) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274149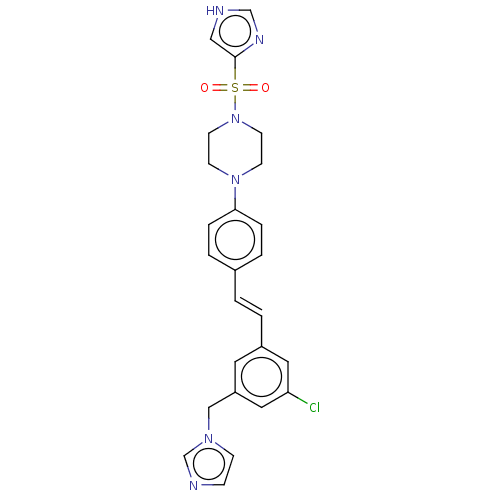 (CHEMBL4127240) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203337 (CHEMBL3980889) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203333 (CHEMBL3968178) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203335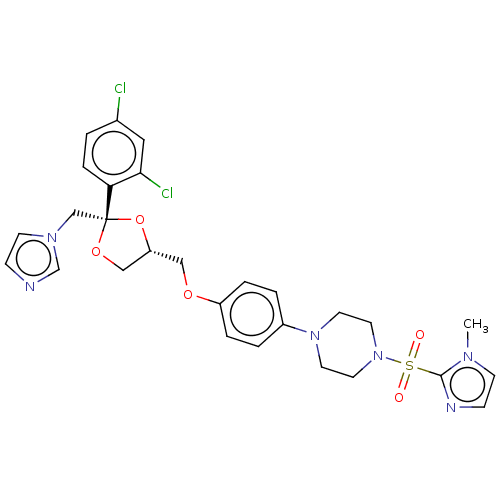 (CHEMBL3891718) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203342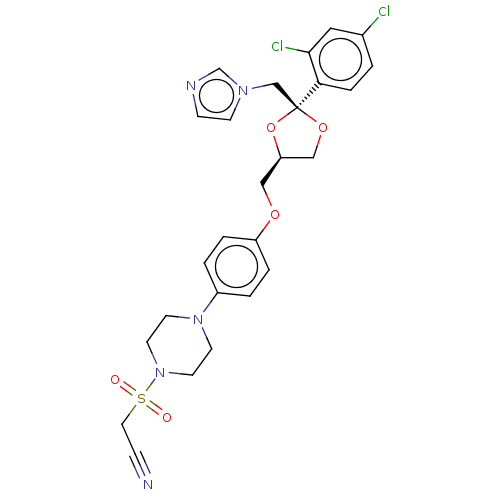 (CHEMBL3952032) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203341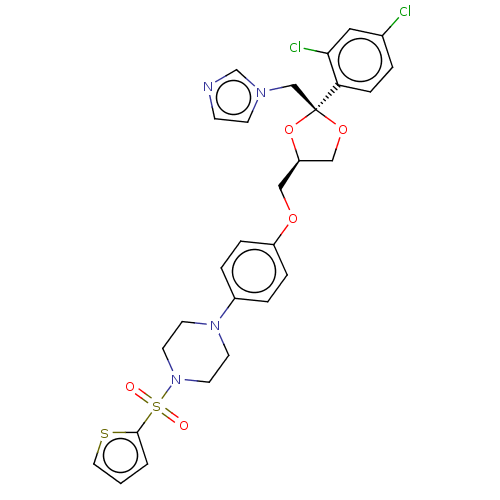 (CHEMBL3960724) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203334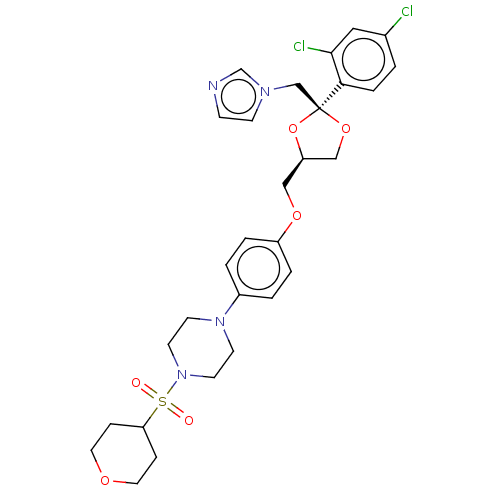 (CHEMBL3931836) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203336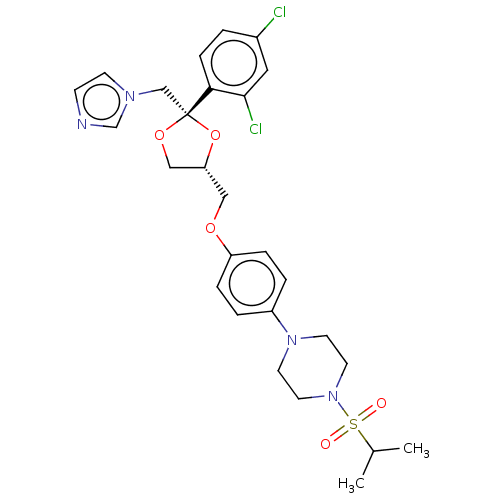 (CHEMBL3894912) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203335 (CHEMBL3891718) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203338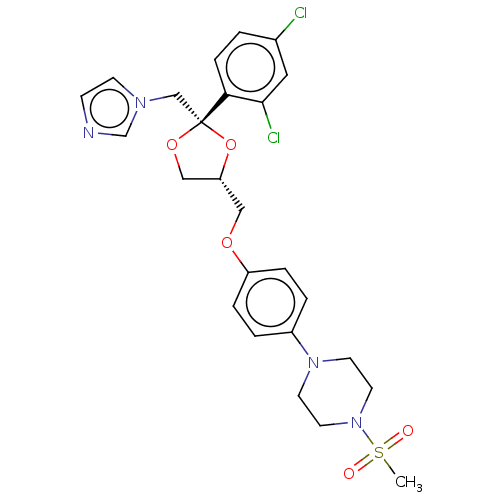 (CHEMBL3983734) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330390 (US9725436, Entry 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274160 (CHEMBL4128621) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50203335 (CHEMBL3891718) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP11B1 (unknown origin) overexpressed in human AD293 cells assessed as reduction in cortisol formation preincubated for 60... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330385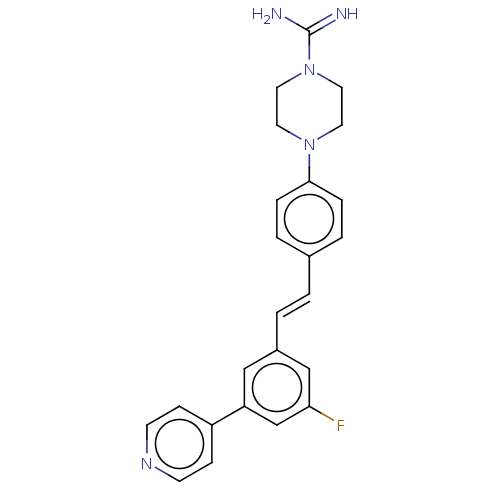 (US9725436, Entry 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203331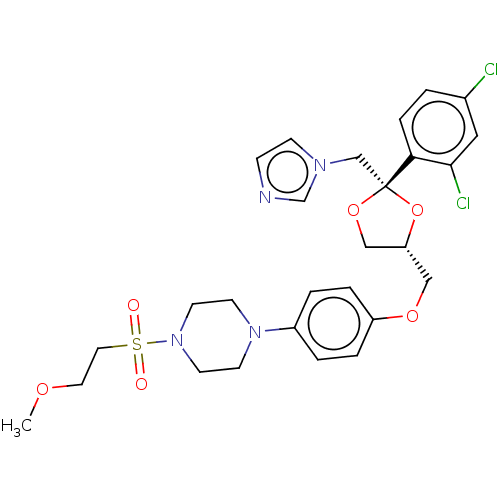 (CHEMBL3964421) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203337 (CHEMBL3980889) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274150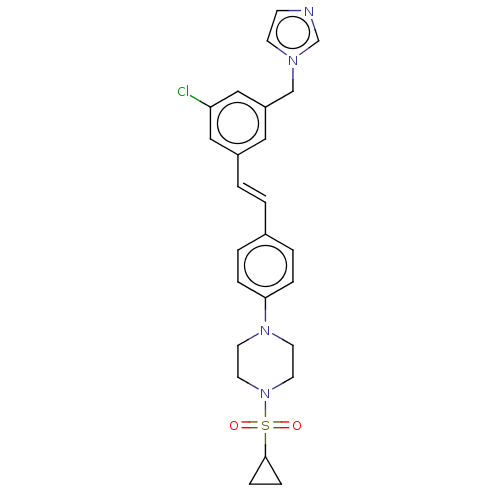 (CHEMBL4127385) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274158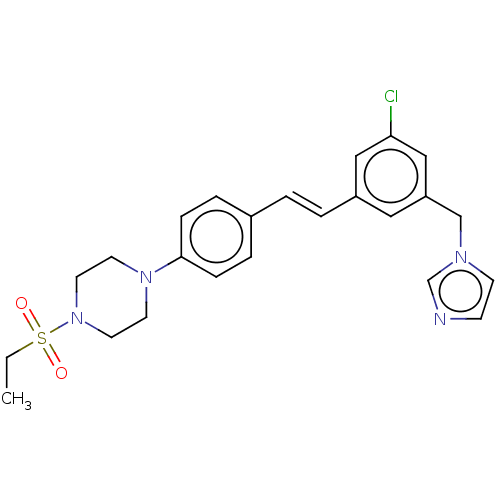 (CHEMBL4128449) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203333 (CHEMBL3968178) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203339 (CHEMBL3922888) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50203333 (CHEMBL3968178) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP11B1 (unknown origin) overexpressed in human AD293 cells assessed as reduction in cortisol formation preincubated for 60... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50203340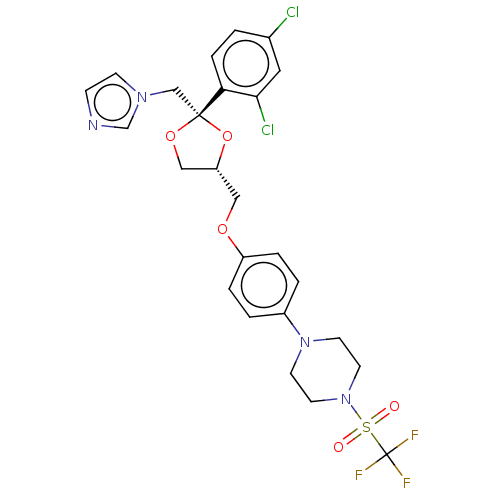 (CHEMBL3938615) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP11B1 (unknown origin) overexpressed in human AD293 cells assessed as reduction in cortisol formation preincubated for 60... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274161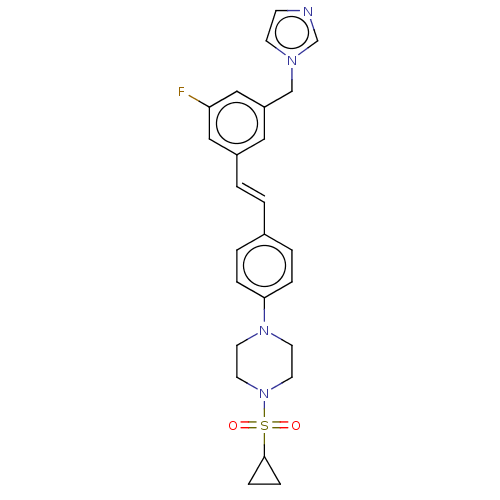 (CHEMBL4125765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330393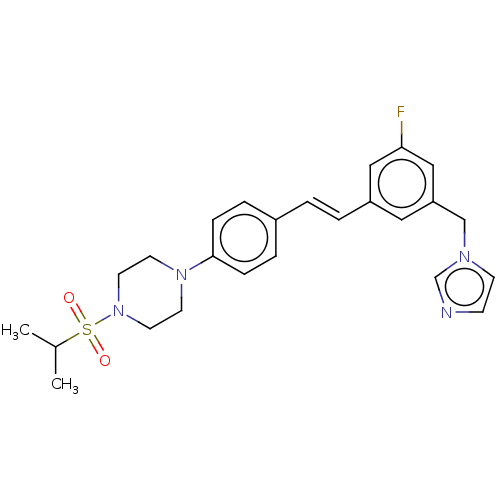 (US9725436, Entry 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50203336 (CHEMBL3894912) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP11B1 (unknown origin) overexpressed in human AD293 cells assessed as reduction in cortisol formation preincubated for 60... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274159 (CHEMBL4129703) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM330396 (US9725436, Entry 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203340 (CHEMBL3938615) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203336 (CHEMBL3894912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 111 total ) | Next | Last >> |