 Found 1090 hits with Last Name = 'yao' and Initial = 'l'
Found 1090 hits with Last Name = 'yao' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271443
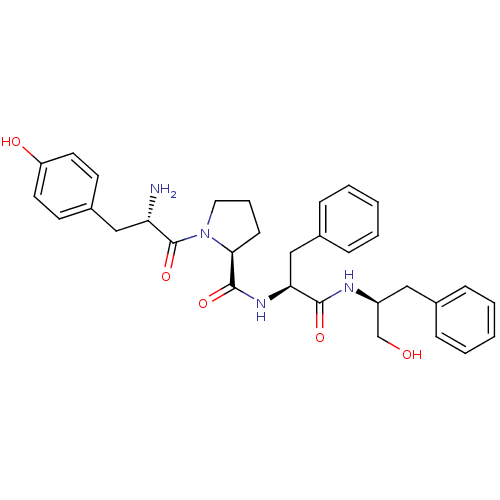
(CHEMBL522293 | Tyr-Pro-Phe-Phe-OCH2OH)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](CO)Cc1ccccc1 |r| Show InChI InChI=1S/C32H38N4O5/c33-27(19-24-13-15-26(38)16-14-24)32(41)36-17-7-12-29(36)31(40)35-28(20-23-10-5-2-6-11-23)30(39)34-25(21-37)18-22-8-3-1-4-9-22/h1-6,8-11,13-16,25,27-29,37-38H,7,12,17-21,33H2,(H,34,39)(H,35,40)/t25-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50163909
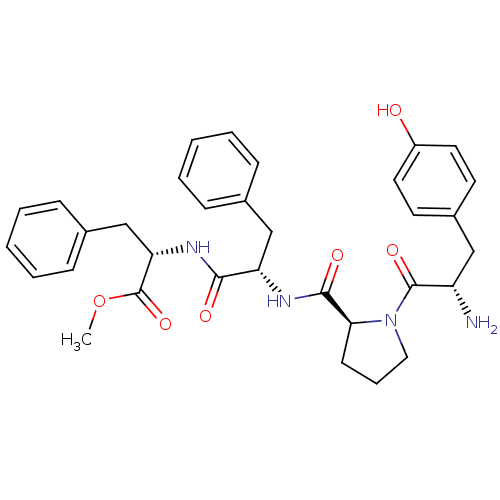
(CHEMBL361922 | Tyr-Pro-Phe-Phe-OCH3 | Tyr-Pro-Phe-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H38N4O6/c1-43-33(42)28(21-23-11-6-3-7-12-23)36-30(39)27(20-22-9-4-2-5-10-22)35-31(40)29-13-8-18-37(29)32(41)26(34)19-24-14-16-25(38)17-15-24/h2-7,9-12,14-17,26-29,38H,8,13,18-21,34H2,1H3,(H,35,40)(H,36,39)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50163913
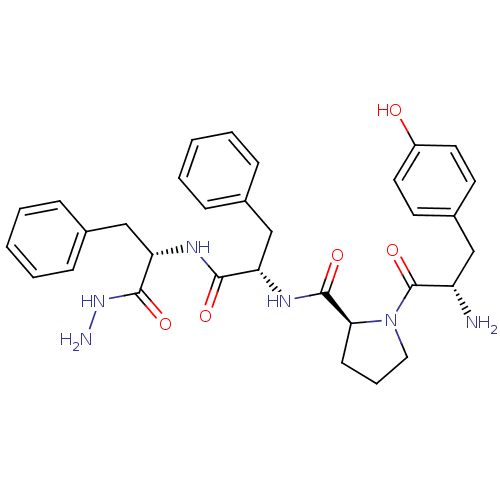
(CHEMBL180777 | Tyr-Pro-Phe-Phe-NHNH2)Show SMILES NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C32H38N6O5/c33-25(18-23-13-15-24(39)16-14-23)32(43)38-17-7-12-28(38)31(42)36-26(19-21-8-3-1-4-9-21)29(40)35-27(30(41)37-34)20-22-10-5-2-6-11-22/h1-6,8-11,13-16,25-28,39H,7,12,17-20,33-34H2,(H,35,40)(H,36,42)(H,37,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271441
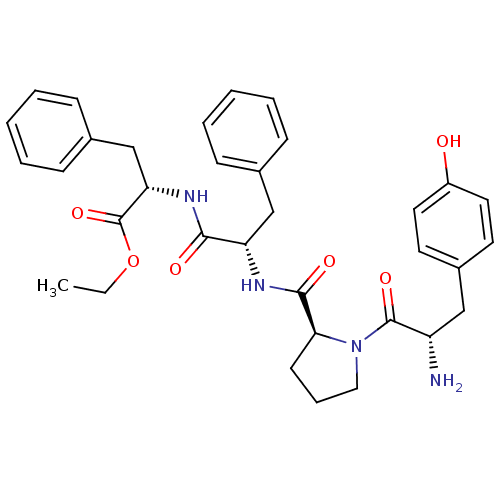
(CHEMBL505502 | Tyr-Pro-Phe-Phe-OCH2CH3)Show SMILES CCOC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C34H40N4O6/c1-2-44-34(43)29(22-24-12-7-4-8-13-24)37-31(40)28(21-23-10-5-3-6-11-23)36-32(41)30-14-9-19-38(30)33(42)27(35)20-25-15-17-26(39)18-16-25/h3-8,10-13,15-18,27-30,39H,2,9,14,19-22,35H2,1H3,(H,36,41)(H,37,40)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271439
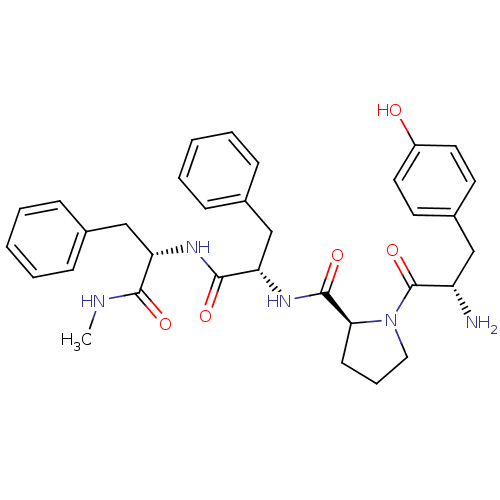
(CHEMBL453689 | Tyr-Pro-Phe-Phe-NHCH3)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H39N5O5/c1-35-30(40)27(20-22-9-4-2-5-10-22)36-31(41)28(21-23-11-6-3-7-12-23)37-32(42)29-13-8-18-38(29)33(43)26(34)19-24-14-16-25(39)17-15-24/h2-7,9-12,14-17,26-29,39H,8,13,18-21,34H2,1H3,(H,35,40)(H,36,41)(H,37,42)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271442
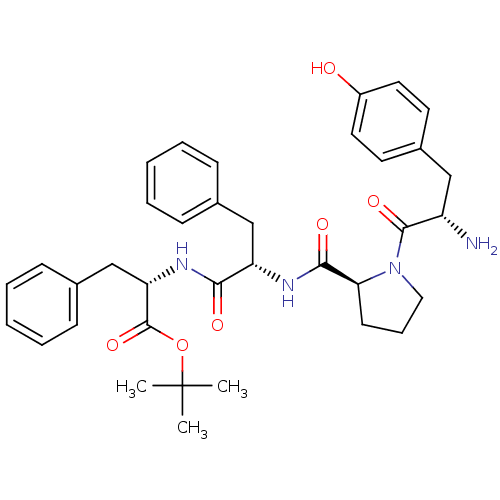
(CHEMBL500195 | Tyr-Pro-Phe-Phe-OC(CH3)3)Show SMILES CC(C)(C)OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C36H44N4O6/c1-36(2,3)46-35(45)30(23-25-13-8-5-9-14-25)39-32(42)29(22-24-11-6-4-7-12-24)38-33(43)31-15-10-20-40(31)34(44)28(37)21-26-16-18-27(41)19-17-26/h4-9,11-14,16-19,28-31,41H,10,15,20-23,37H2,1-3H3,(H,38,43)(H,39,42)/t28-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271442

(CHEMBL500195 | Tyr-Pro-Phe-Phe-OC(CH3)3)Show SMILES CC(C)(C)OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C36H44N4O6/c1-36(2,3)46-35(45)30(23-25-13-8-5-9-14-25)39-32(42)29(22-24-11-6-4-7-12-24)38-33(43)31-15-10-20-40(31)34(44)28(37)21-26-16-18-27(41)19-17-26/h4-9,11-14,16-19,28-31,41H,10,15,20-23,37H2,1-3H3,(H,38,43)(H,39,42)/t28-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271440

(CHEMBL505975 | Tyr-Pro-Phe-Phe-N(CH3)2)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C34H41N5O5/c1-38(2)34(44)29(22-24-12-7-4-8-13-24)37-31(41)28(21-23-10-5-3-6-11-23)36-32(42)30-14-9-19-39(30)33(43)27(35)20-25-15-17-26(40)18-16-25/h3-8,10-13,15-18,27-30,40H,9,14,19-22,35H2,1-2H3,(H,36,42)(H,37,41)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271441

(CHEMBL505502 | Tyr-Pro-Phe-Phe-OCH2CH3)Show SMILES CCOC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C34H40N4O6/c1-2-44-34(43)29(22-24-12-7-4-8-13-24)37-31(40)28(21-23-10-5-3-6-11-23)36-32(41)30-14-9-19-38(30)33(42)27(35)20-25-15-17-26(39)18-16-25/h3-8,10-13,15-18,27-30,39H,2,9,14,19-22,35H2,1H3,(H,36,41)(H,37,40)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271439

(CHEMBL453689 | Tyr-Pro-Phe-Phe-NHCH3)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H39N5O5/c1-35-30(40)27(20-22-9-4-2-5-10-22)36-31(41)28(21-23-11-6-3-7-12-23)37-32(42)29-13-8-18-38(29)33(43)26(34)19-24-14-16-25(39)17-15-24/h2-7,9-12,14-17,26-29,39H,8,13,18-21,34H2,1H3,(H,35,40)(H,36,41)(H,37,42)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50163909

(CHEMBL361922 | Tyr-Pro-Phe-Phe-OCH3 | Tyr-Pro-Phe-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H38N4O6/c1-43-33(42)28(21-23-11-6-3-7-12-23)36-30(39)27(20-22-9-4-2-5-10-22)35-31(40)29-13-8-18-37(29)32(41)26(34)19-24-14-16-25(38)17-15-24/h2-7,9-12,14-17,26-29,38H,8,13,18-21,34H2,1H3,(H,35,40)(H,36,39)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271440

(CHEMBL505975 | Tyr-Pro-Phe-Phe-N(CH3)2)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C34H41N5O5/c1-38(2)34(44)29(22-24-12-7-4-8-13-24)37-31(41)28(21-23-10-5-3-6-11-23)36-32(42)30-14-9-19-39(30)33(43)27(35)20-25-15-17-26(40)18-16-25/h3-8,10-13,15-18,27-30,40H,9,14,19-22,35H2,1-2H3,(H,36,42)(H,37,41)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50163913

(CHEMBL180777 | Tyr-Pro-Phe-Phe-NHNH2)Show SMILES NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C32H38N6O5/c33-25(18-23-13-15-24(39)16-14-23)32(43)38-17-7-12-28(38)31(42)36-26(19-21-8-3-1-4-9-21)29(40)35-27(30(41)37-34)20-22-10-5-2-6-11-22/h1-6,8-11,13-16,25-28,39H,7,12,17-20,33-34H2,(H,35,40)(H,36,42)(H,37,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271443

(CHEMBL522293 | Tyr-Pro-Phe-Phe-OCH2OH)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](CO)Cc1ccccc1 |r| Show InChI InChI=1S/C32H38N4O5/c33-27(19-24-13-15-26(38)16-14-24)32(41)36-17-7-12-29(36)31(40)35-28(20-23-10-5-2-6-11-23)30(39)34-25(21-37)18-22-8-3-1-4-9-22/h1-6,8-11,13-16,25,27-29,37-38H,7,12,17-21,33H2,(H,34,39)(H,35,40)/t25-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50462728
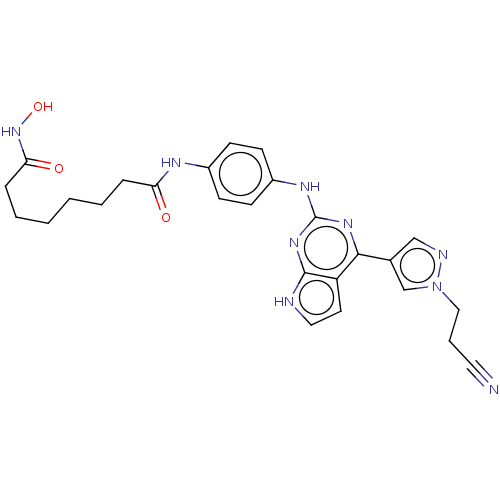
(CHEMBL4242626)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cnn(CCC#N)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C26H29N9O3/c27-13-5-15-35-17-18(16-29-35)24-21-12-14-28-25(21)33-26(32-24)31-20-10-8-19(9-11-20)30-22(36)6-3-1-2-4-7-23(37)34-38/h8-12,14,16-17,38H,1-7,15H2,(H,30,36)(H,34,37)(H2,28,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]ATP |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50462726
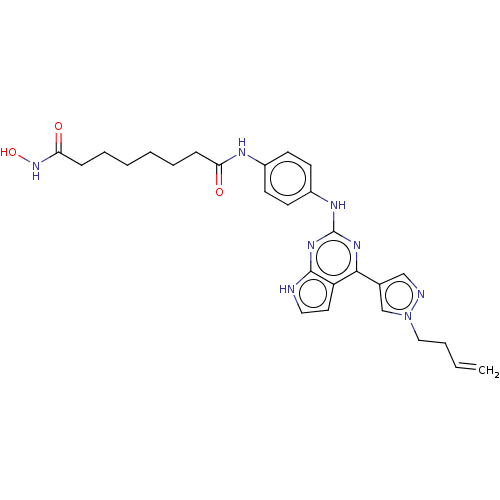
(CHEMBL4247128)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cnn(CCC=C)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C27H32N8O3/c1-2-3-16-35-18-19(17-29-35)25-22-14-15-28-26(22)33-27(32-25)31-21-12-10-20(11-13-21)30-23(36)8-6-4-5-7-9-24(37)34-38/h2,10-15,17-18,38H,1,3-9,16H2,(H,30,36)(H,34,37)(H2,28,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]ATP |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]ATP |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] as substrate in presence of [gamma-33P]-ATP |
J Med Chem 60: 8336-8357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00678
BindingDB Entry DOI: 10.7270/Q2CN76B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50250140

(CHEMBL4098975)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(NCCCn2cc(cn2)-c2ncnc3[nH]ccc23)cc1 Show InChI InChI=1S/C26H32N8O3/c35-23(6-3-1-2-4-7-24(36)33-37)32-21-10-8-20(9-11-21)27-13-5-15-34-17-19(16-31-34)25-22-12-14-28-26(22)30-18-29-25/h8-12,14,16-18,27,37H,1-7,13,15H2,(H,32,35)(H,33,36)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected insect cells using RHKKAc as substrate in pres... |
J Med Chem 60: 8336-8357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00678
BindingDB Entry DOI: 10.7270/Q2CN76B6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50462723
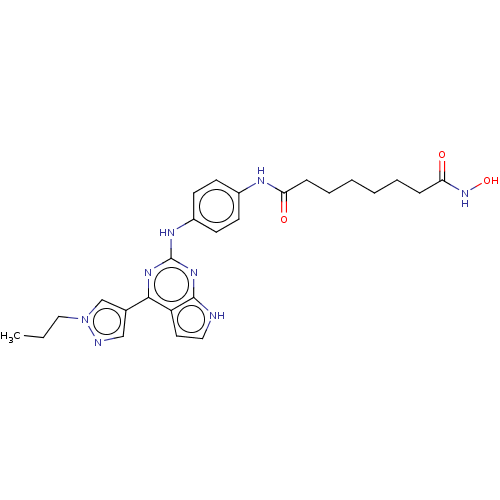
(CHEMBL4243458)Show SMILES CCCn1cc(cn1)-c1nc(Nc2ccc(NC(=O)CCCCCCC(=O)NO)cc2)nc2[nH]ccc12 Show InChI InChI=1S/C26H32N8O3/c1-2-15-34-17-18(16-28-34)24-21-13-14-27-25(21)32-26(31-24)30-20-11-9-19(10-12-20)29-22(35)7-5-3-4-6-8-23(36)33-37/h9-14,16-17,37H,2-8,15H2,1H3,(H,29,35)(H,33,36)(H2,27,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]ATP |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50462724

(CHEMBL4243347)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cn[nH]c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C23H26N8O3/c32-19(5-3-1-2-4-6-20(33)31-34)27-16-7-9-17(10-8-16)28-23-29-21(15-13-25-26-14-15)18-11-12-24-22(18)30-23/h7-14,34H,1-6H2,(H,25,26)(H,27,32)(H,31,33)(H2,24,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using p53 (379 to 382 residues) derived fluorogenic peptide RHKKAc as substrate |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50462726

(CHEMBL4247128)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cnn(CCC=C)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C27H32N8O3/c1-2-3-16-35-18-19(17-29-35)25-22-14-15-28-26(22)33-27(32-25)31-21-12-10-20(11-13-21)30-23(36)8-6-4-5-7-9-24(37)34-38/h2,10-15,17-18,38H,1,3-9,16H2,(H,30,36)(H,34,37)(H2,28,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using p53 (379 to 382 residues) derived fluorogenic peptide RHKKAc as substrate |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50462728

(CHEMBL4242626)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cnn(CCC#N)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C26H29N9O3/c27-13-5-15-35-17-18(16-29-35)24-21-12-14-28-25(21)33-26(32-24)31-20-10-8-19(9-11-20)30-22(36)6-3-1-2-4-7-23(37)34-38/h8-12,14,16-17,38H,1-7,15H2,(H,30,36)(H,34,37)(H2,28,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using p53 (379 to 382 residues) derived fluorogenic peptide RHKKAc as substrate |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50250142

(CHEMBL4091233)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(CCCn2cc(cn2)-c2ncnc3[nH]ccc23)cc1 Show InChI InChI=1S/C26H31N7O3/c34-23(7-3-1-2-4-8-24(35)32-36)31-21-11-9-19(10-12-21)6-5-15-33-17-20(16-30-33)25-22-13-14-27-26(22)29-18-28-25/h9-14,16-18,36H,1-8,15H2,(H,31,34)(H,32,35)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected insect cells using RHKKAc as substrate in pres... |
J Med Chem 60: 8336-8357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00678
BindingDB Entry DOI: 10.7270/Q2CN76B6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50462724

(CHEMBL4243347)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cn[nH]c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C23H26N8O3/c32-19(5-3-1-2-4-6-20(33)31-34)27-16-7-9-17(10-8-16)28-23-29-21(15-13-25-26-14-15)18-11-12-24-22(18)30-23/h7-14,34H,1-6H2,(H,25,26)(H,27,32)(H,31,33)(H2,24,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]ATP |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50324585
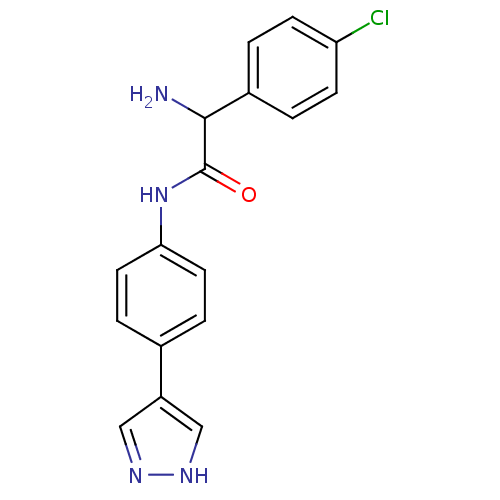
(CHEMBL1215022 | N-(4-(1H-Pyrazol-4-yl)phenyl)-2-am...)Show SMILES NC(C(=O)Nc1ccc(cc1)-c1cn[nH]c1)c1ccc(Cl)cc1 Show InChI InChI=1S/C17H15ClN4O/c18-14-5-1-12(2-6-14)16(19)17(23)22-15-7-3-11(4-8-15)13-9-20-21-10-13/h1-10,16H,19H2,(H,20,21)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
J Med Chem 53: 5727-37 (2010)
Article DOI: 10.1021/jm100579r
BindingDB Entry DOI: 10.7270/Q2833S8C |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50324574
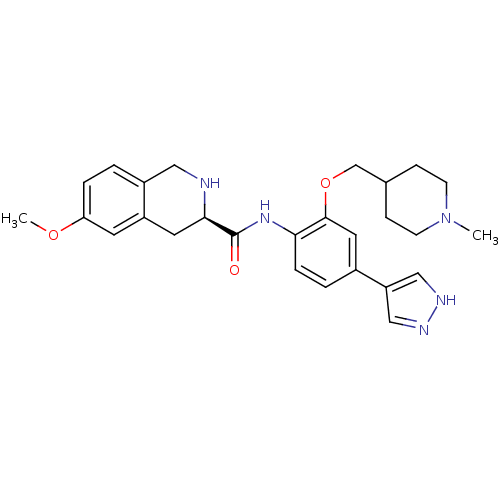
((R)-6-Methoxy-N-(2-((1-methylpiperidin-4-yl)methox...)Show SMILES COc1ccc2CN[C@H](Cc2c1)C(=O)Nc1ccc(cc1OCC1CCN(C)CC1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C27H33N5O3/c1-32-9-7-18(8-10-32)17-35-26-13-19(22-15-29-30-16-22)4-6-24(26)31-27(33)25-12-21-11-23(34-2)5-3-20(21)14-28-25/h3-6,11,13,15-16,18,25,28H,7-10,12,14,17H2,1-2H3,(H,29,30)(H,31,33)/t25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
J Med Chem 53: 5727-37 (2010)
Article DOI: 10.1021/jm100579r
BindingDB Entry DOI: 10.7270/Q2833S8C |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273535
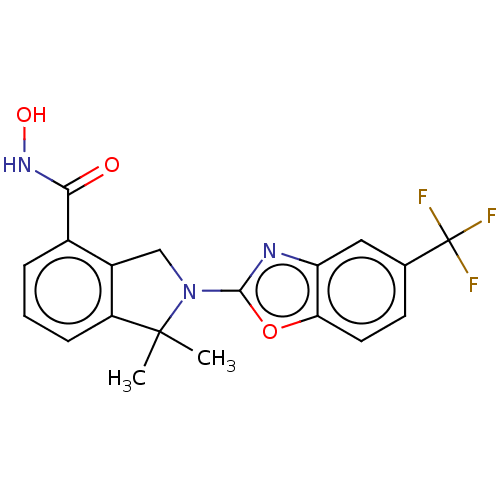
(CHEMBL4130288 | US11535607, Example 18-1)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1nc2cc(ccc2o1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O3/c1-18(2)13-5-3-4-11(16(26)24-27)12(13)9-25(18)17-23-14-8-10(19(20,21)22)6-7-15(14)28-17/h3-8,27H,9H2,1-2H3,(H,24,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma33P]-ATP |
Bioorg Med Chem Lett 28: 1357-1362 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.009
BindingDB Entry DOI: 10.7270/Q2FB55KP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM383860
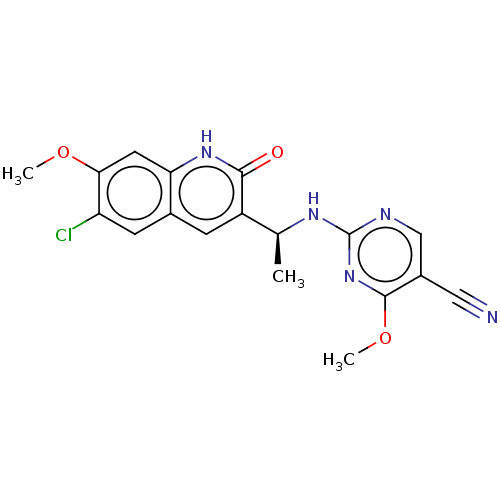
(US10280150, Cmpd No I-143 | US10550099, Compound I...)Show SMILES COc1cc2[nH]c(=O)c(cc2cc1Cl)[C@H](C)Nc1ncc(C#N)c(OC)n1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Forma Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Myc-DDK-tagged IDH1 R132H mutant expressed in human U87MG cells assessed as reduction in 2-HG levels after 24 hrs by RapidFire hi... |
J Med Chem 62: 6575-6596 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00362
BindingDB Entry DOI: 10.7270/Q2VD72TR |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406127
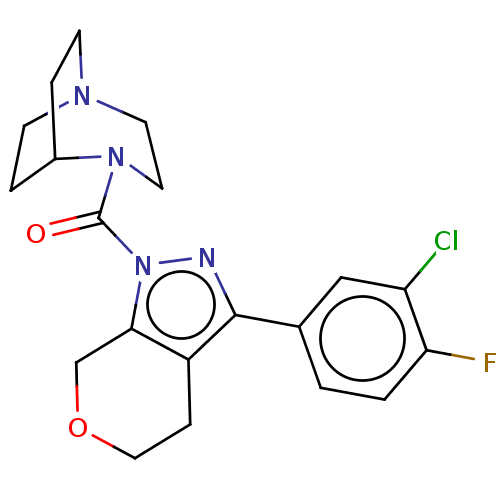
(CHEMBL5290844)Show SMILES Cc1cc(C)c(CCCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H28FO5P/c1-14-9-15(2)19(20(10-14)17-6-7-21(23)16(3)11-17)5-4-8-29(27,28)13-18(24)12-22(25)26/h6-7,9-11,27-29H,4-5,8,12-13H2,1-3H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50250136
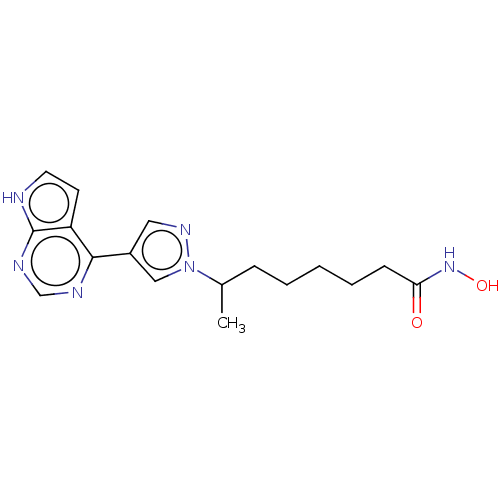
(CHEMBL4095596)Show InChI InChI=1S/C17H22N6O2/c1-12(5-3-2-4-6-15(24)22-25)23-10-13(9-21-23)16-14-7-8-18-17(14)20-11-19-16/h7-12,25H,2-6H2,1H3,(H,22,24)(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected insect cells using RHKKAc as substrate in pres... |
J Med Chem 60: 8336-8357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00678
BindingDB Entry DOI: 10.7270/Q2CN76B6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50250135

(CHEMBL4060201)Show InChI InChI=1S/C16H20N6O2/c23-14(21-24)5-3-1-2-4-8-22-10-12(9-20-22)15-13-6-7-17-16(13)19-11-18-15/h6-7,9-11,24H,1-5,8H2,(H,21,23)(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected insect cells using RHKKAc as substrate in pres... |
J Med Chem 60: 8336-8357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00678
BindingDB Entry DOI: 10.7270/Q2CN76B6 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50614520
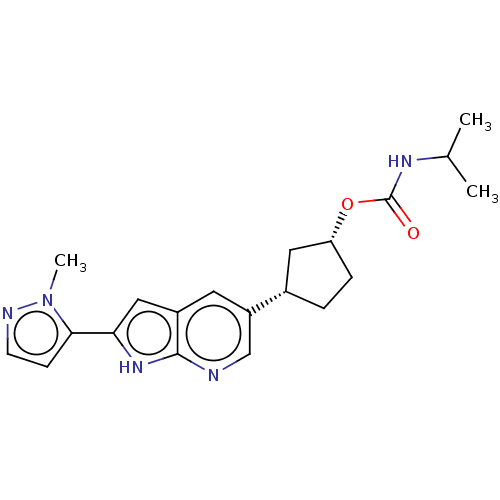
(CHEMBL5271228)Show SMILES CC(C)NC(=O)O[C@@H]1CC[C@@H](C1)c1cnc2[nH]c(cc2c1)-c1ccnn1C |r| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50462723

(CHEMBL4243458)Show SMILES CCCn1cc(cn1)-c1nc(Nc2ccc(NC(=O)CCCCCCC(=O)NO)cc2)nc2[nH]ccc12 Show InChI InChI=1S/C26H32N8O3/c1-2-15-34-17-18(16-28-34)24-21-13-14-27-25(21)32-26(31-24)30-20-11-9-19(10-12-20)29-22(35)7-5-3-4-6-8-23(36)33-37/h9-14,16-17,37H,2-8,15H2,1H3,(H,29,35)(H,33,36)(H2,27,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using p53 (379 to 382 residues) derived fluorogenic peptide RHKKAc as substrate |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50250144

(CHEMBL4069283)Show InChI InChI=1S/C17H22N6O2/c24-15(22-25)6-4-2-1-3-5-9-23-11-13(10-21-23)16-14-7-8-18-17(14)20-12-19-16/h7-8,10-12,25H,1-6,9H2,(H,22,24)(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected insect cells using RHKKAc as substrate in pres... |
J Med Chem 60: 8336-8357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00678
BindingDB Entry DOI: 10.7270/Q2CN76B6 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50324604
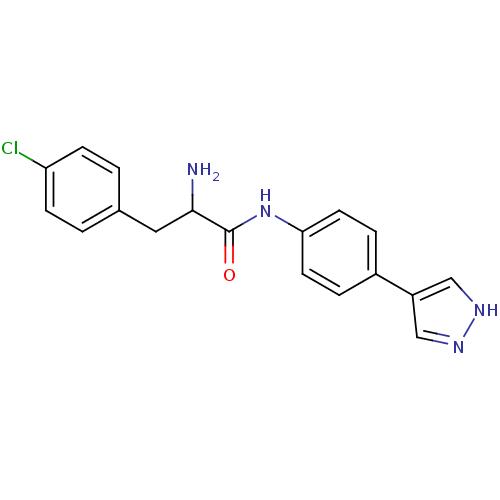
(CHEMBL1215021 | N-(4-(1H-Pyrazol-4-yl)phenyl)-2-am...)Show SMILES NC(Cc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1)-c1cn[nH]c1 Show InChI InChI=1S/C18H17ClN4O/c19-15-5-1-12(2-6-15)9-17(20)18(24)23-16-7-3-13(4-8-16)14-10-21-22-11-14/h1-8,10-11,17H,9,20H2,(H,21,22)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
J Med Chem 53: 5727-37 (2010)
Article DOI: 10.1021/jm100579r
BindingDB Entry DOI: 10.7270/Q2833S8C |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50503280

(CHEMBL4469055 | US11311527, Cpd ID I-26 | US113762...)Show SMILES C[C@H](Nc1ccc(C#N)n(C)c1=O)c1cc2cc(Cl)c(O[C@H](C)c3ccccn3)cc2[nH]c1=O |r| Show InChI InChI=1S/C25H22ClN5O3/c1-14(29-21-8-7-17(13-27)31(3)25(21)33)18-10-16-11-19(26)23(12-22(16)30-24(18)32)34-15(2)20-6-4-5-9-28-20/h4-12,14-15,29H,1-3H3,(H,30,32)/t14-,15+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDH1 R132H mutant in human HCT116 cells assessed as reduction in 2-HG levels after 24 hrs by RapidFire high-throughput mass spectrometr... |
J Med Chem 63: 1612-1623 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01423
BindingDB Entry DOI: 10.7270/Q2J106F7 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50324592
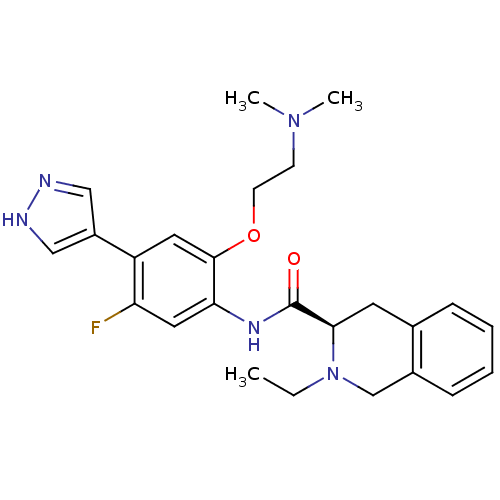
((R)-N-(2-(2-(Dimethylamino)ethoxy)-5-fluoro-4-(1H-...)Show SMILES CCN1Cc2ccccc2C[C@@H]1C(=O)Nc1cc(F)c(cc1OCCN(C)C)-c1cn[nH]c1 |r| Show InChI InChI=1S/C25H30FN5O2/c1-4-31-16-18-8-6-5-7-17(18)11-23(31)25(32)29-22-13-21(26)20(19-14-27-28-15-19)12-24(22)33-10-9-30(2)3/h5-8,12-15,23H,4,9-11,16H2,1-3H3,(H,27,28)(H,29,32)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
J Med Chem 53: 5727-37 (2010)
Article DOI: 10.1021/jm100579r
BindingDB Entry DOI: 10.7270/Q2833S8C |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50503280

(CHEMBL4469055 | US11311527, Cpd ID I-26 | US113762...)Show SMILES C[C@H](Nc1ccc(C#N)n(C)c1=O)c1cc2cc(Cl)c(O[C@H](C)c3ccccn3)cc2[nH]c1=O |r| Show InChI InChI=1S/C25H22ClN5O3/c1-14(29-21-8-7-17(13-27)31(3)25(21)33)18-10-16-11-19(26)23(12-22(16)30-24(18)32)34-15(2)20-6-4-5-9-28-20/h4-12,14-15,29H,1-3H3,(H,30,32)/t14-,15+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDH1 R132C mutant in human HCT116 cells assessed as reduction in 2-HG levels after 24 hrs by RapidFire high-throughput mass spectrometr... |
J Med Chem 63: 1612-1623 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01423
BindingDB Entry DOI: 10.7270/Q2J106F7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50503263
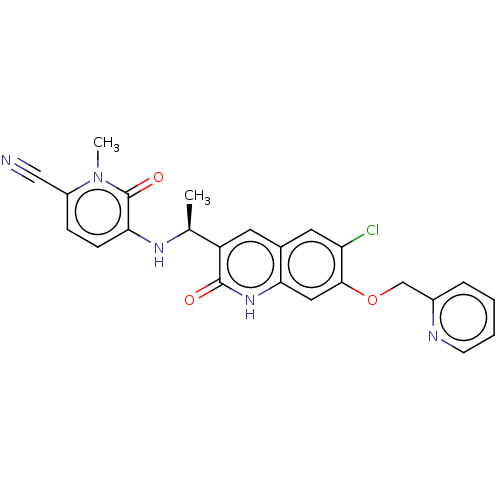
(CHEMBL4464313 | US11311527, Cpd ID I-23 | US113762...)Show SMILES C[C@H](Nc1ccc(C#N)n(C)c1=O)c1cc2cc(Cl)c(OCc3ccccn3)cc2[nH]c1=O |r| Show InChI InChI=1S/C24H20ClN5O3/c1-14(28-20-7-6-17(12-26)30(2)24(20)32)18-9-15-10-19(25)22(11-21(15)29-23(18)31)33-13-16-5-3-4-8-27-16/h3-11,14,28H,13H2,1-2H3,(H,29,31)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDH1 R132H mutant in human HCT116 cells assessed as reduction in 2-HG levels after 24 hrs by RapidFire high-throughput mass spectrometr... |
J Med Chem 63: 1612-1623 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01423
BindingDB Entry DOI: 10.7270/Q2J106F7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50503260
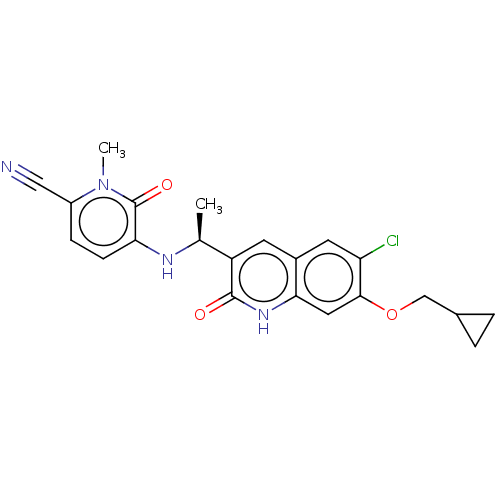
(CHEMBL4456610 | US11311527, Cpd ID I-27 | US113762...)Show SMILES C[C@H](Nc1ccc(C#N)n(C)c1=O)c1cc2cc(Cl)c(OCC3CC3)cc2[nH]c1=O |r| Show InChI InChI=1S/C22H21ClN4O3/c1-12(25-18-6-5-15(10-24)27(2)22(18)29)16-7-14-8-17(23)20(30-11-13-3-4-13)9-19(14)26-21(16)28/h5-9,12-13,25H,3-4,11H2,1-2H3,(H,26,28)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDH1 R132H mutant in human HCT116 cells assessed as reduction in 2-HG levels after 24 hrs by RapidFire high-throughput mass spectrometr... |
J Med Chem 63: 1612-1623 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01423
BindingDB Entry DOI: 10.7270/Q2J106F7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273522
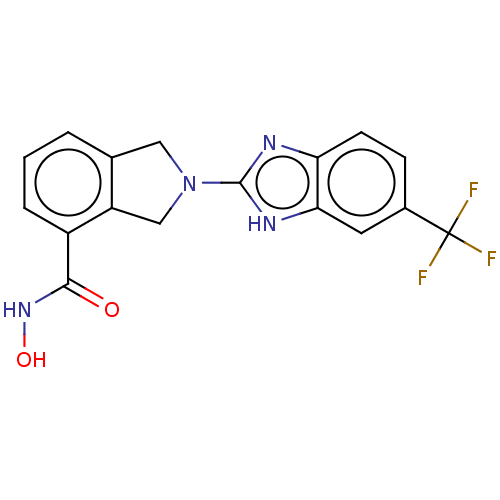
(CHEMBL4127743 | US11535607, Example 12-1)Show SMILES ONC(=O)c1cccc2CN(Cc12)c1nc2ccc(cc2[nH]1)C(F)(F)F Show InChI InChI=1S/C17H13F3N4O2/c18-17(19,20)10-4-5-13-14(6-10)22-16(21-13)24-7-9-2-1-3-11(12(9)8-24)15(25)23-26/h1-6,26H,7-8H2,(H,21,22)(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50273537
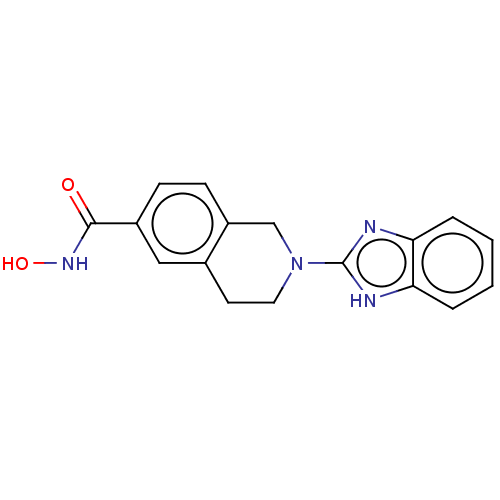
(CHEMBL4128164 | US11535607, Example 50-5)Show InChI InChI=1S/C17H16N4O2/c22-16(20-23)12-5-6-13-10-21(8-7-11(13)9-12)17-18-14-3-1-2-4-15(14)19-17/h1-6,9,23H,7-8,10H2,(H,18,19)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273553
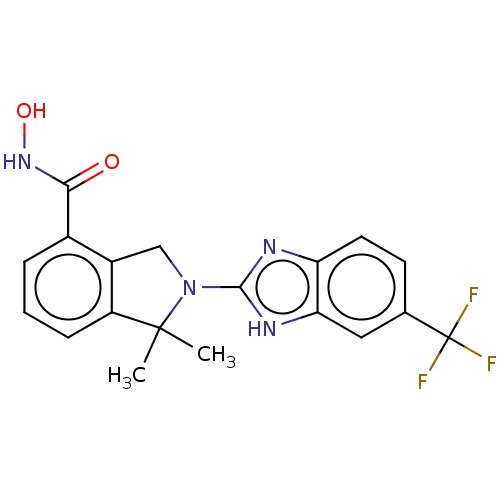
(CHEMBL4127020 | US10508088, ID HDTK028 | US1153560...)Show SMILES CC1(C)N(Cc2c1cccc2C(=O)NO)c1nc2ccc(cc2[nH]1)C(F)(F)F Show InChI InChI=1S/C19H17F3N4O2/c1-18(2)13-5-3-4-11(16(27)25-28)12(13)9-26(18)17-23-14-7-6-10(19(20,21)22)8-15(14)24-17/h3-8,28H,9H2,1-2H3,(H,23,24)(H,25,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50273525
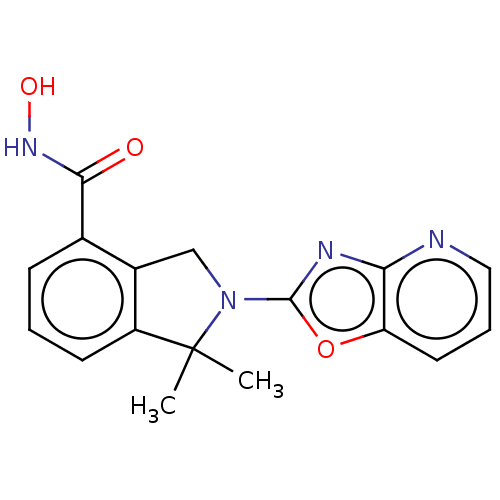
(CHEMBL4128972 | US11535607, Example 18-2)Show InChI InChI=1S/C17H16N4O3/c1-17(2)12-6-3-5-10(15(22)20-23)11(12)9-21(17)16-19-14-13(24-16)7-4-8-18-14/h3-8,23H,9H2,1-2H3,(H,20,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... |
Bioorg Med Chem Lett 28: 2143-2147 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.021
BindingDB Entry DOI: 10.7270/Q2PR7ZGX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50462724

(CHEMBL4243347)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cn[nH]c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C23H26N8O3/c32-19(5-3-1-2-4-6-20(33)31-34)27-16-7-9-17(10-8-16)28-23-29-21(15-13-25-26-14-15)18-11-12-24-22(18)30-23/h7-14,34H,1-6H2,(H,25,26)(H,27,32)(H,31,33)(H2,24,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 using p53 (379 to 382 residues) derived fluorogenic peptide RHKKAc as substrate |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data