

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12210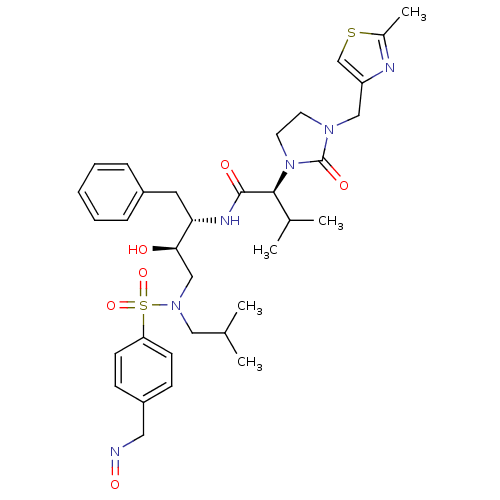 ((2S)-N-[(2S,3R)-3-hydroxy-4-({4-[(1E)-(hydroxyimin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12213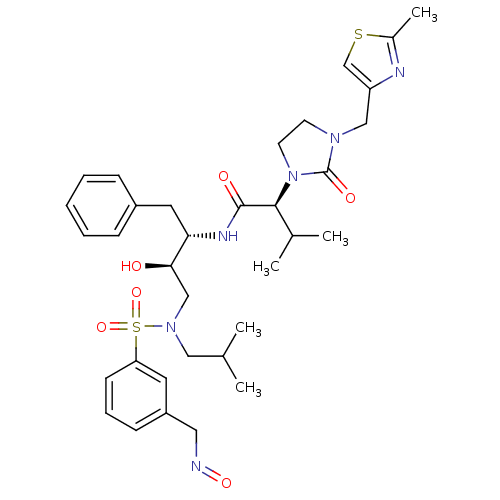 ((2S)-N-[(2S,3R)-3-hydroxy-4-({3-[(1E)-(hydroxyimin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12209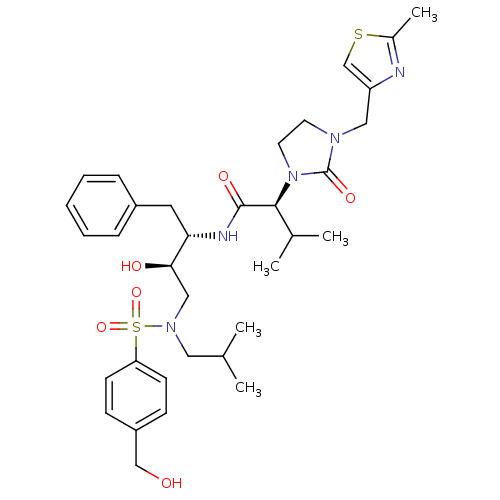 ((2S)-N-[(2S,3R)-3-hydroxy-4-{[4-(hydroxymethyl)ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12198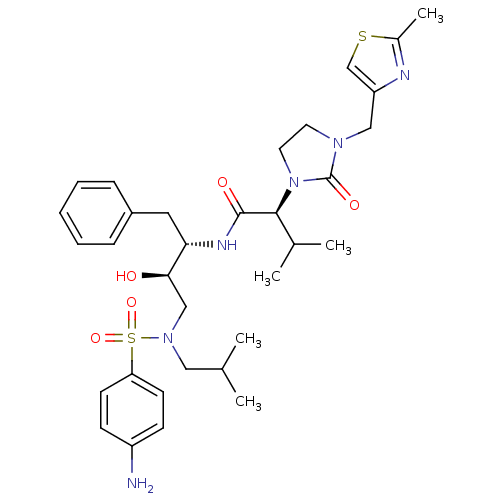 ((2S)-N-[(2S,3R)-4-[(4-aminobenzene)(2-methylpropyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12203 ((2S)-N-[(2S,3R)-4-[(4-acetylbenzene)(2-methylpropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12212 ((2S)-N-[(2S,3R)-3-hydroxy-4-{[3-(hydroxymethyl)ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12208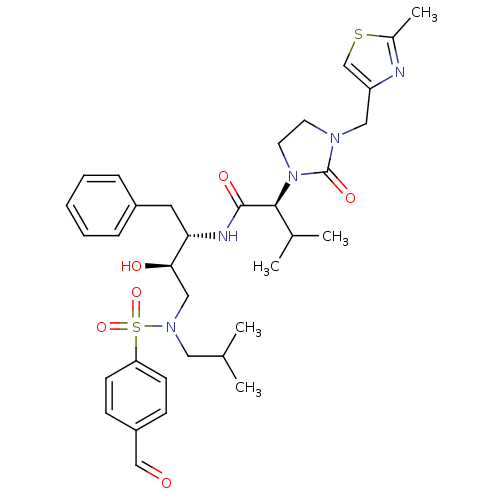 ((2S)-N-[(2S,3R)-4-[(4-formylbenzene)(2-methylpropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12207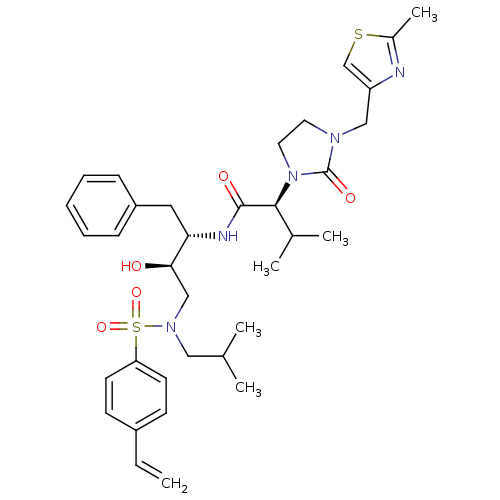 ((2S)-N-[(2S,3R)-4-[(4-ethenylbenzene)(2-methylprop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12206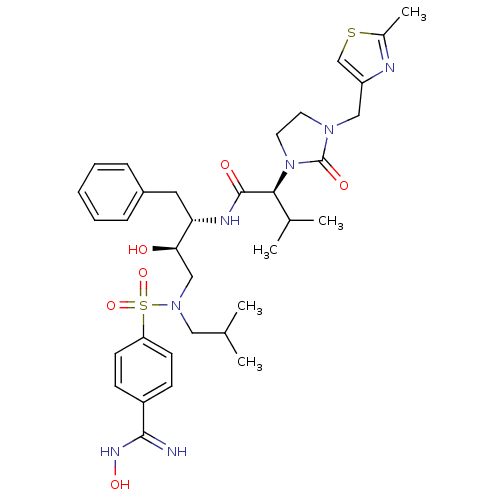 ((2S)-N-[(2S,3R)-3-hydroxy-4-{[4-(N'-hydroxycarbami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12204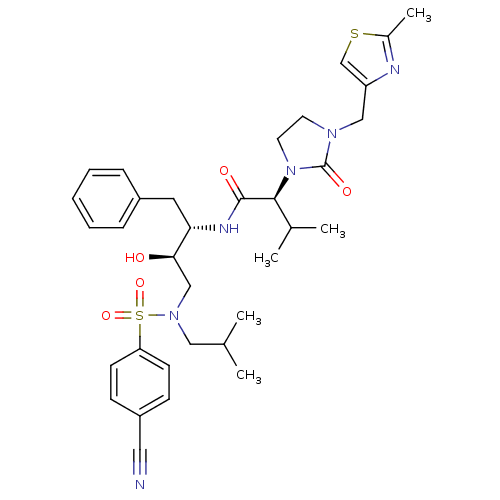 ((2S)-N-[(2S,3R)-4-[(4-cyanobenzene)(2-methylpropyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12199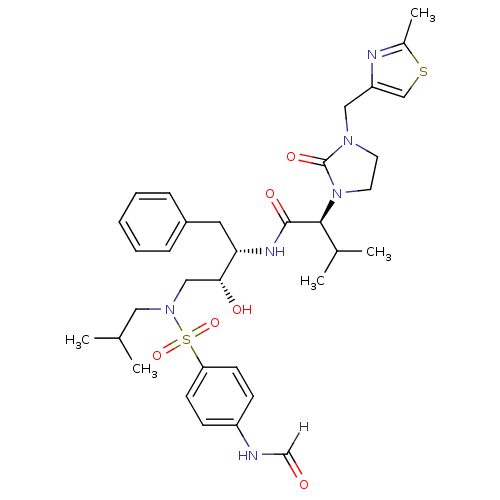 ((2S)-N-[(2S,3R)-4-[(4-formamidobenzene)(2-methylpr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227798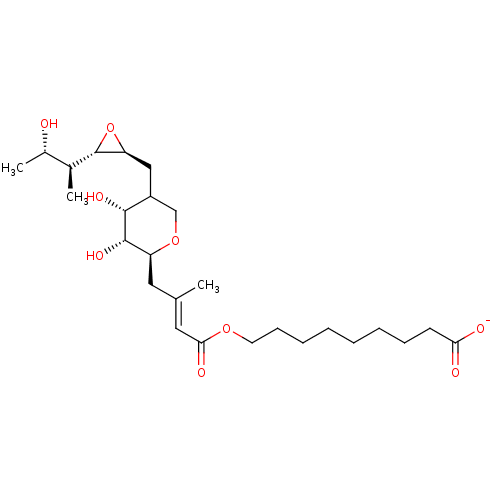 (CHEMBL42269) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12205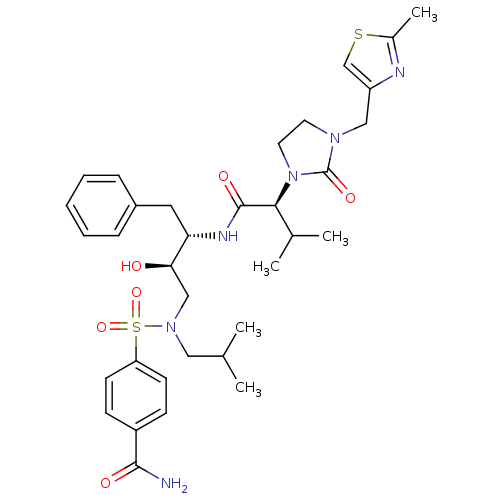 (4-{[(2R,3S)-2-hydroxy-3-[(2S)-3-methyl-2-{3-[(2-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12201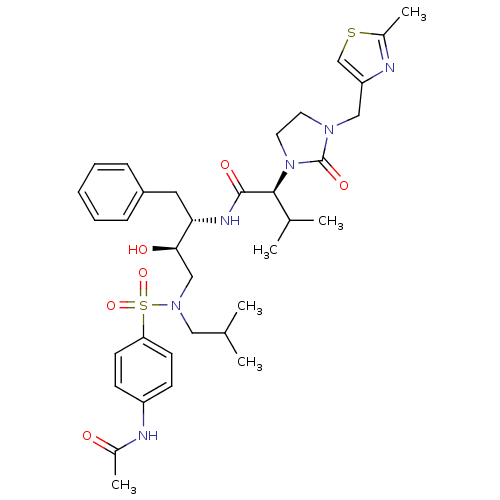 ((2S)-N-[(2S,3R)-4-[(4-acetamidobenzene)(2-methylpr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Rattus norvegicus (Rat)) | BDBM50438747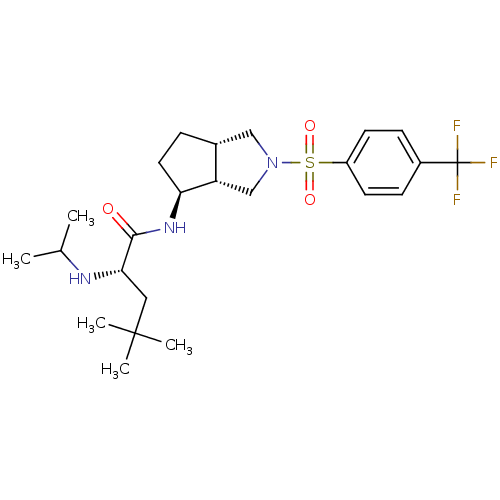 (CHEMBL2414787) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064, United States. xenia.b.searle@abbvie.com Curated by ChEMBL | Assay Description Inhibition of rat N-type Cav2.2 channel expressed in HEK293 cells under hyperpolarized condition by whole cell patch-clamp manual electrophysiology a... | Bioorg Med Chem Lett 23: 4857-61 (2013) Article DOI: 10.1016/j.bmcl.2013.06.074 BindingDB Entry DOI: 10.7270/Q2280915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227812 (CHEMBL288953) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12211 ((2S)-N-[(2S,3R)-3-hydroxy-4-({4-[(1E)-(methoxyimin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 653 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Rattus norvegicus (Rat)) | BDBM50438746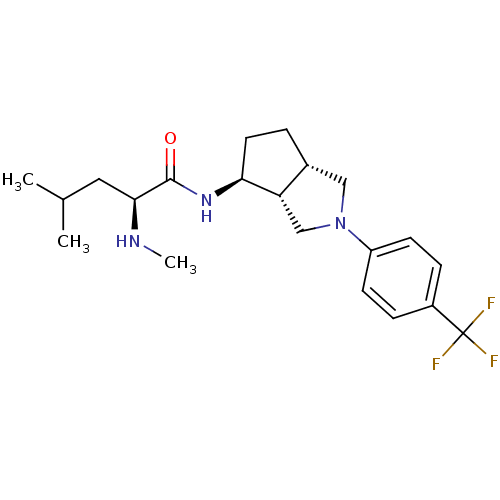 (CHEMBL2414771) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064, United States. xenia.b.searle@abbvie.com Curated by ChEMBL | Assay Description Inhibition of rat N-type Cav2.2 channel expressed in HEK293 cells under depolarized condition by whole cell patch-clamp manual electrophysiology assa... | Bioorg Med Chem Lett 23: 4857-61 (2013) Article DOI: 10.1016/j.bmcl.2013.06.074 BindingDB Entry DOI: 10.7270/Q2280915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227796 (CHEMBL38412) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12200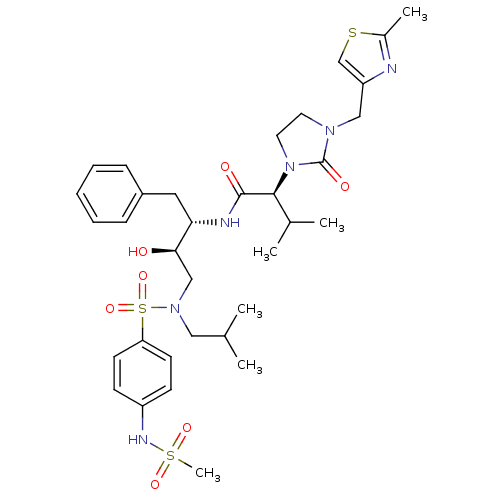 ((2S)-N-[(2S,3R)-3-hydroxy-4-[(4-methanesulfonamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227800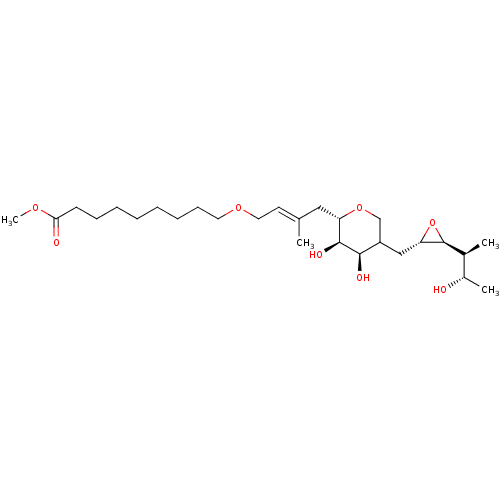 (CHEMBL40757) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227811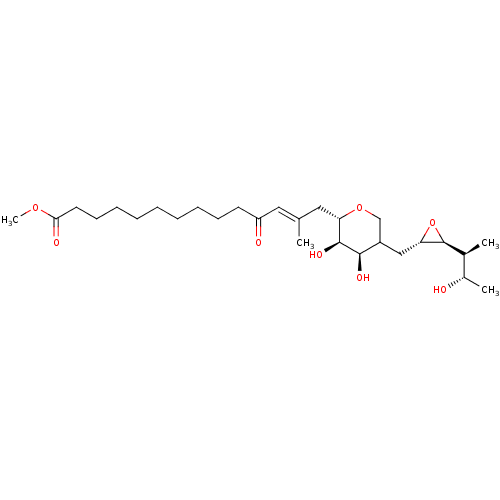 (CHEMBL287840) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Rattus norvegicus (Rat)) | BDBM50438746 (CHEMBL2414771) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064, United States. xenia.b.searle@abbvie.com Curated by ChEMBL | Assay Description Inhibition of rat N-type Cav2.2 channel expressed in HEK293 cells under hyperpolarized condition by whole cell patch-clamp manual electrophysiology a... | Bioorg Med Chem Lett 23: 4857-61 (2013) Article DOI: 10.1016/j.bmcl.2013.06.074 BindingDB Entry DOI: 10.7270/Q2280915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) assessed as dextromethorphan O-demethylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) assessed as tolbutamide hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) assessed as phenacetin O-deethylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) assessed as S-mephenytion hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227797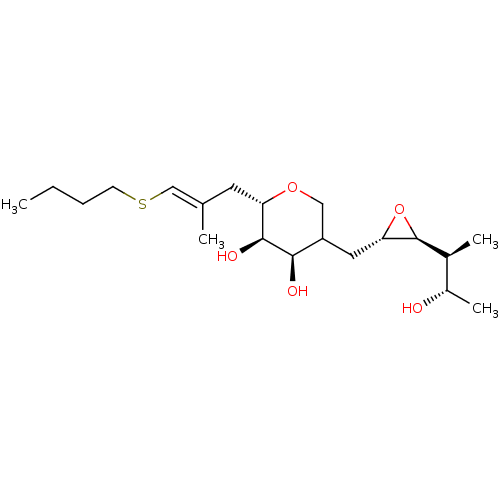 (CHEMBL42195) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) assessed as dextromethorphan O-demethylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) assessed as S-mephenytion hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) assessed as tolbutamide hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) assessed as phenacetin O-deethylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12202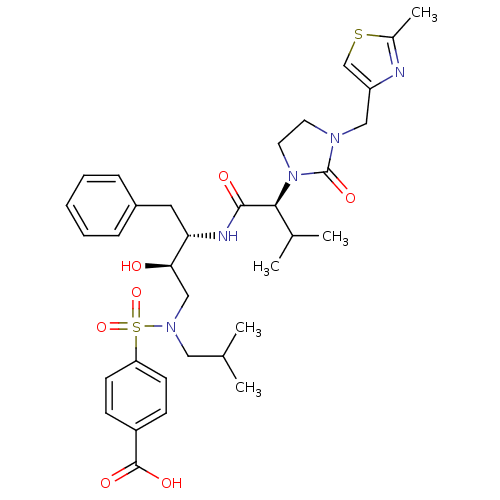 (4-{[(2R,3S)-2-hydroxy-3-[(2S)-3-methyl-2-{3-[(2-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Rattus norvegicus (Rat)) | BDBM50438747 (CHEMBL2414787) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064, United States. xenia.b.searle@abbvie.com Curated by ChEMBL | Assay Description Inhibition of rat N-type Cav2.2 channel expressed in HEK293 cells under depolarized condition by whole cell patch-clamp manual electrophysiology assa... | Bioorg Med Chem Lett 23: 4857-61 (2013) Article DOI: 10.1016/j.bmcl.2013.06.074 BindingDB Entry DOI: 10.7270/Q2280915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227810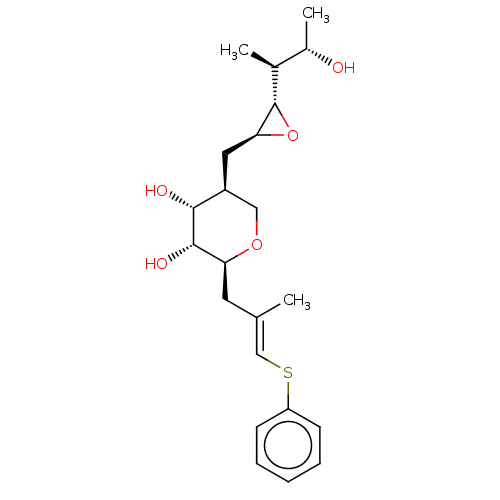 (CHEMBL2115370) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227803 (CHEMBL40920) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227808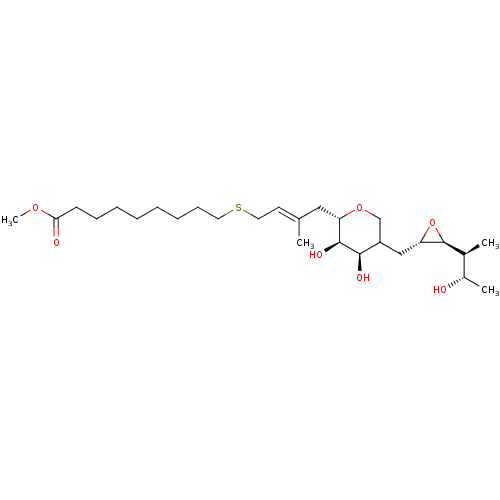 (CHEMBL41447) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227795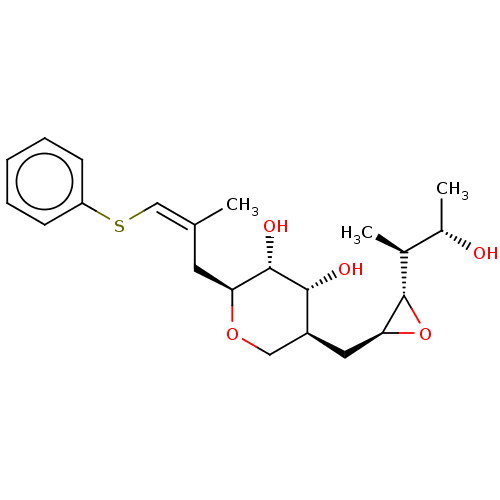 (CHEMBL2114438) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227807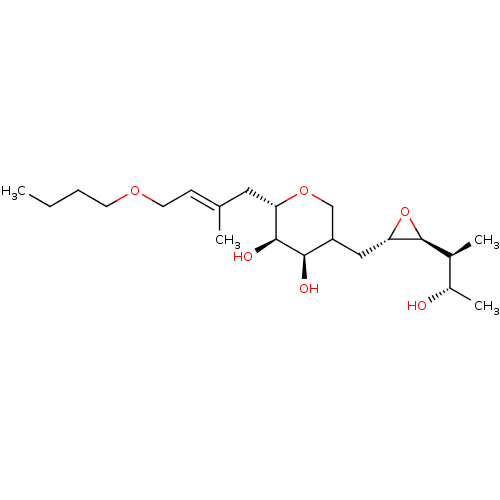 (CHEMBL431719) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 5.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227801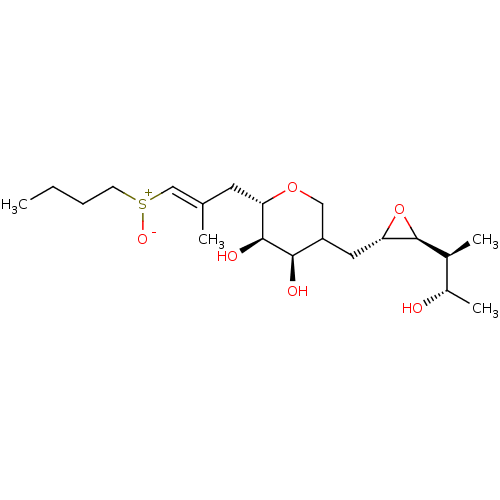 (CHEMBL290861) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Escherichia coli) | BDBM50227802 (CHEMBL412877) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Isoleucyl-tRNA synthetase | J Med Chem 32: 151-60 (1989) BindingDB Entry DOI: 10.7270/Q28054TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |