 Found 1530 hits with Last Name = 'yi' and Initial = 'c'
Found 1530 hits with Last Name = 'yi' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328391
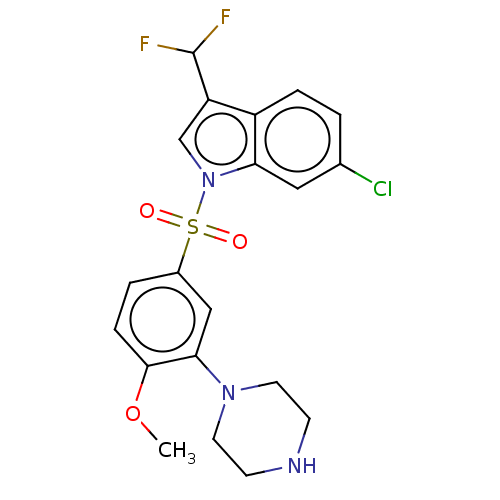
(6-chloro-3-(difluoromethyl)-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(Cl)cc12 Show InChI InChI=1S/C20H20ClF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286349
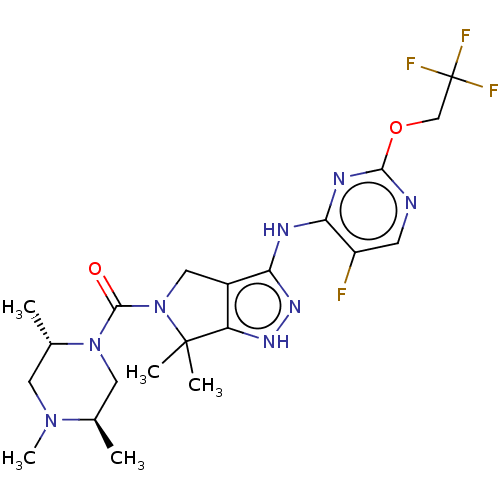
(US11220518, Ex. No. K7 | US11780853, Example K7 | ...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C21H28F4N8O2/c1-11-8-32(12(2)7-31(11)5)19(34)33-9-13-15(20(33,3)4)29-30-16(13)27-17-14(22)6-26-18(28-17)35-10-21(23,24)25/h6,11-12H,7-10H2,1-5H3,(H2,26,27,28,29,30)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM286349

(US11220518, Ex. No. K7 | US11780853, Example K7 | ...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C21H28F4N8O2/c1-11-8-32(12(2)7-31(11)5)19(34)33-9-13-15(20(33,3)4)29-30-16(13)27-17-14(22)6-26-18(28-17)35-10-21(23,24)25/h6,11-12H,7-10H2,1-5H3,(H2,26,27,28,29,30)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US9518060 (2016)
BindingDB Entry DOI: 10.7270/Q2ZK5JPN |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286349

(US11220518, Ex. No. K7 | US11780853, Example K7 | ...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C21H28F4N8O2/c1-11-8-32(12(2)7-31(11)5)19(34)33-9-13-15(20(33,3)4)29-30-16(13)27-17-14(22)6-26-18(28-17)35-10-21(23,24)25/h6,11-12H,7-10H2,1-5H3,(H2,26,27,28,29,30)/t11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592784
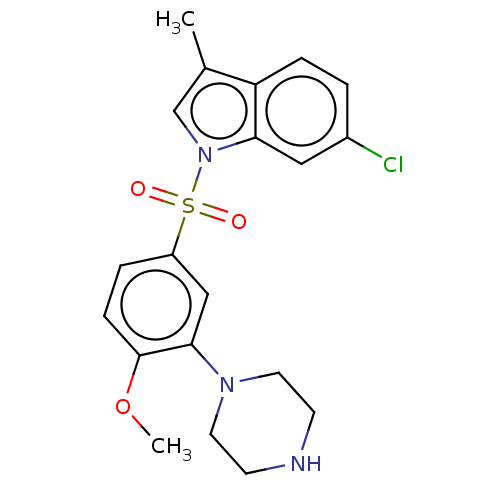
(CHEMBL5177311)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Cl)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286350

(US11220518, Ex. No. K8 | US11780853, Example K8 | ...)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C22H30F4N8O2/c1-12-8-32(6)20(2,3)10-33(12)19(35)34-9-13-15(21(34,4)5)30-31-16(13)28-17-14(23)7-27-18(29-17)36-11-22(24,25)26/h7,12H,8-11H2,1-6H3,(H2,27,28,29,30,31)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM286350

(US11220518, Ex. No. K8 | US11780853, Example K8 | ...)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C22H30F4N8O2/c1-12-8-32(6)20(2,3)10-33(12)19(35)34-9-13-15(21(34,4)5)30-31-16(13)28-17-14(23)7-27-18(29-17)36-11-22(24,25)26/h7,12H,8-11H2,1-6H3,(H2,27,28,29,30,31)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US9518060 (2016)
BindingDB Entry DOI: 10.7270/Q2ZK5JPN |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286350

(US11220518, Ex. No. K8 | US11780853, Example K8 | ...)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C22H30F4N8O2/c1-12-8-32(6)20(2,3)10-33(12)19(35)34-9-13-15(21(34,4)5)30-31-16(13)28-17-14(23)7-27-18(29-17)36-11-22(24,25)26/h7,12H,8-11H2,1-6H3,(H2,27,28,29,30,31)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]p-Cl-DPDPE from rat delta opioid receptor expressed in rat C6 cells |
Bioorg Med Chem Lett 19: 4603-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.093
BindingDB Entry DOI: 10.7270/Q2WQ04RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328379
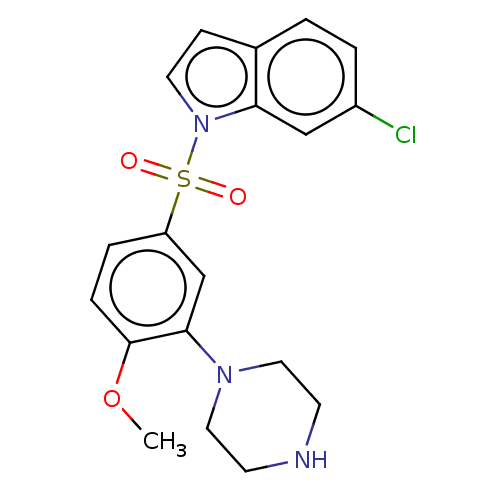
(6-chloro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(Cl)cc12 Show InChI InChI=1S/C19H20ClN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592785
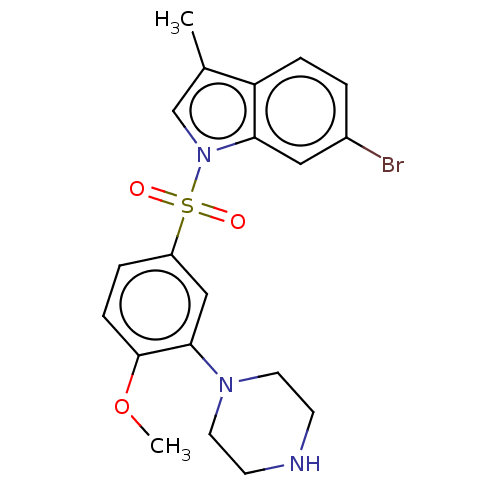
(CHEMBL5206617)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Br)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328389

(3-(difluoromethyl)-6-fluoro-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(F)cc12 Show InChI InChI=1S/C20H20F3N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130285
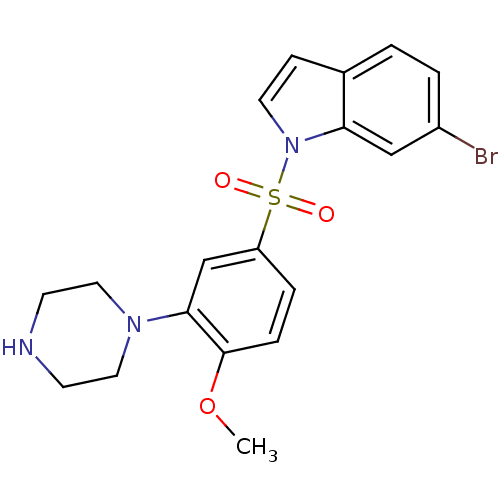
(6-Bromo-1-(4-methoxy-3-piperazin-1-yl-benzenesulfo...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(Br)cc12 Show InChI InChI=1S/C19H20BrN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328393

(3-(difluoromethyl)-6-bromo-1-((4-methoxy-3-(pipera...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(Br)cc12 Show InChI InChI=1S/C20H20BrF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286348
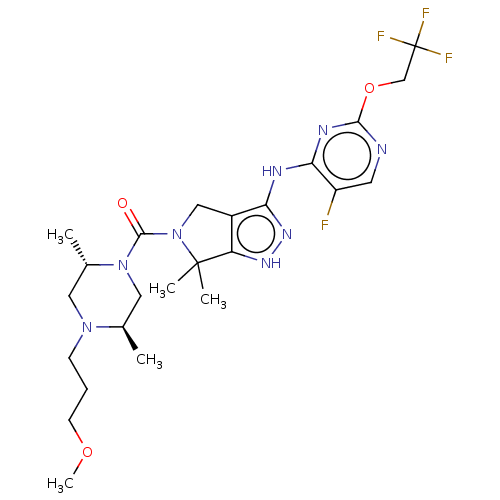
(US11220518, Ex. No. K6 | US11780853, Example K6 | ...)Show SMILES COCCCN1C[C@H](C)N(C[C@H]1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C24H34F4N8O3/c1-14-11-35(15(2)10-34(14)7-6-8-38-5)22(37)36-12-16-18(23(36,3)4)32-33-19(16)30-20-17(25)9-29-21(31-20)39-13-24(26,27)28/h9,14-15H,6-8,10-13H2,1-5H3,(H2,29,30,31,32,33)/t14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286348

(US11220518, Ex. No. K6 | US11780853, Example K6 | ...)Show SMILES COCCCN1C[C@H](C)N(C[C@H]1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C24H34F4N8O3/c1-14-11-35(15(2)10-34(14)7-6-8-38-5)22(37)36-12-16-18(23(36,3)4)32-33-19(16)30-20-17(25)9-29-21(31-20)39-13-24(26,27)28/h9,14-15H,6-8,10-13H2,1-5H3,(H2,29,30,31,32,33)/t14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM286348

(US11220518, Ex. No. K6 | US11780853, Example K6 | ...)Show SMILES COCCCN1C[C@H](C)N(C[C@H]1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C24H34F4N8O3/c1-14-11-35(15(2)10-34(14)7-6-8-38-5)22(37)36-12-16-18(23(36,3)4)32-33-19(16)30-20-17(25)9-29-21(31-20)39-13-24(26,27)28/h9,14-15H,6-8,10-13H2,1-5H3,(H2,29,30,31,32,33)/t14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.376 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US9518060 (2016)
BindingDB Entry DOI: 10.7270/Q2ZK5JPN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394084

(US9974785, Example 3)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C19H18ClF2N3O2S/c20-14-4-1-2-7-17(14)28(26,27)25-12-13(19(21)22)18-15(5-3-6-16(18)25)24-10-8-23-9-11-24/h1-7,12,19,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116950
BindingDB Entry DOI: 10.7270/Q2CC14NC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328408
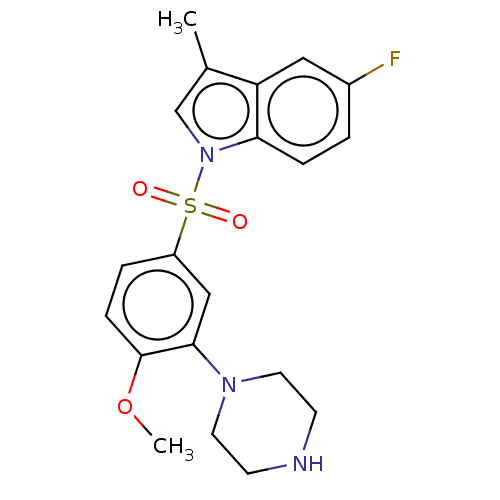
(5-fluoro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2cc(F)ccc12 Show InChI InChI=1S/C20H22FN3O3S/c1-14-13-24(18-5-3-15(21)11-17(14)18)28(25,26)16-4-6-20(27-2)19(12-16)23-9-7-22-8-10-23/h3-6,11-13,22H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328383
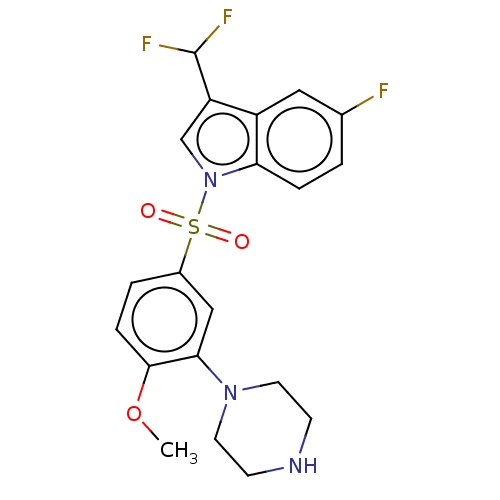
(3-(difluoromethyl)-5-fluoro-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2cc(F)ccc12 Show InChI InChI=1S/C20H20F3N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-10-13(21)2-4-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394083

(US9974785, Example 2)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H18F3N3O2S/c20-14-4-1-2-7-17(14)28(26,27)25-12-13(19(21)22)18-15(5-3-6-16(18)25)24-10-8-23-9-11-24/h1-7,12,19,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116950
BindingDB Entry DOI: 10.7270/Q2CC14NC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50590516
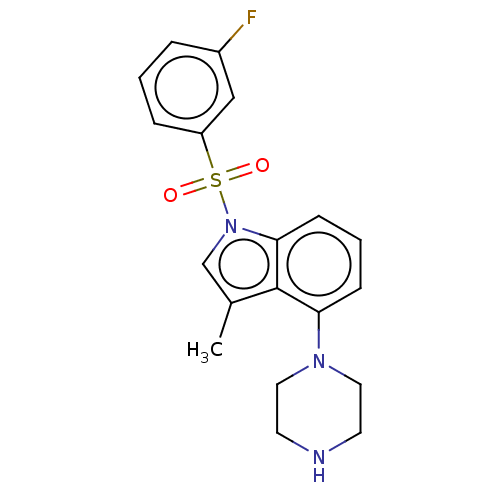
(CHEMBL5196479)Show SMILES Cc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(F)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116950
BindingDB Entry DOI: 10.7270/Q2CC14NC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394086

(US9974785, Example 5)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C19H18F3N3O2S/c20-13-3-1-4-14(11-13)28(26,27)25-12-15(19(21)22)18-16(5-2-6-17(18)25)24-9-7-23-8-10-24/h1-6,11-12,19,23H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116950
BindingDB Entry DOI: 10.7270/Q2CC14NC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328387

(5-bromo-3-(difluoromethyl)-1-((4-methoxy-3-(pipera...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2cc(Br)ccc12 Show InChI InChI=1S/C20H20BrF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-10-13(21)2-4-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328385
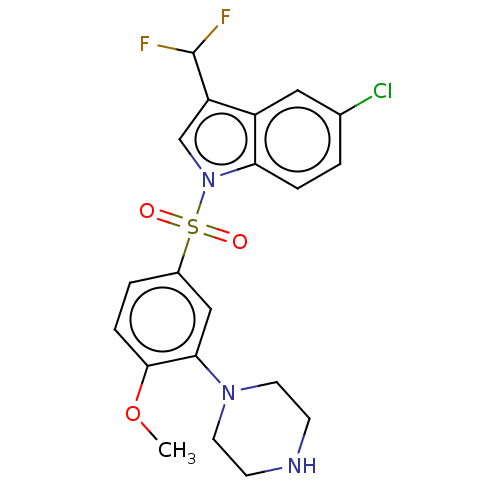
(5-chloro-3-(difluoromethyl)-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2cc(Cl)ccc12 Show InChI InChI=1S/C20H20ClF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-10-13(21)2-4-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328409
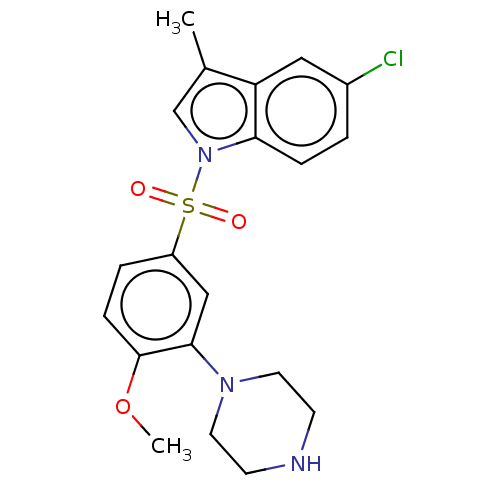
(5-chloro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2cc(Cl)ccc12 Show InChI InChI=1S/C20H22ClN3O3S/c1-14-13-24(18-5-3-15(21)11-17(14)18)28(25,26)16-4-6-20(27-2)19(12-16)23-9-7-22-8-10-23/h3-6,11-13,22H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394087
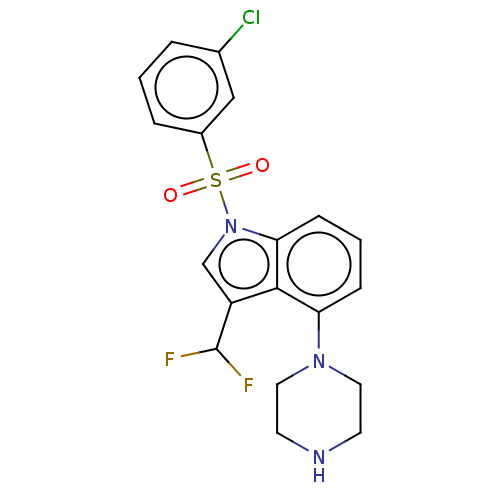
(US9974785, Example 6)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H18ClF2N3O2S/c20-13-3-1-4-14(11-13)28(26,27)25-12-15(19(21)22)18-16(5-2-6-17(18)25)24-9-7-23-8-10-24/h1-6,11-12,19,23H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116950
BindingDB Entry DOI: 10.7270/Q2CC14NC |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM286347

(US11220518, Ex. No. K5 | US11780853, Example K5 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C24H37FN8O3/c1-7-36-22-26-11-18(25)21(28-22)27-20-17-14-33(24(4,5)19(17)29-30-20)23(34)32-13-15(2)31(12-16(32)3)9-8-10-35-6/h11,15-16H,7-10,12-14H2,1-6H3,(H2,26,27,28,29,30)/t15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US9518060 (2016)
BindingDB Entry DOI: 10.7270/Q2ZK5JPN |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286347

(US11220518, Ex. No. K5 | US11780853, Example K5 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C24H37FN8O3/c1-7-36-22-26-11-18(25)21(28-22)27-20-17-14-33(24(4,5)19(17)29-30-20)23(34)32-13-15(2)31(12-16(32)3)9-8-10-35-6/h11,15-16H,7-10,12-14H2,1-6H3,(H2,26,27,28,29,30)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286347

(US11220518, Ex. No. K5 | US11780853, Example K5 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C24H37FN8O3/c1-7-36-22-26-11-18(25)21(28-22)27-20-17-14-33(24(4,5)19(17)29-30-20)23(34)32-13-15(2)31(12-16(32)3)9-8-10-35-6/h11,15-16H,7-10,12-14H2,1-6H3,(H2,26,27,28,29,30)/t15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116950
BindingDB Entry DOI: 10.7270/Q2CC14NC |
More data for this
Ligand-Target Pair | |
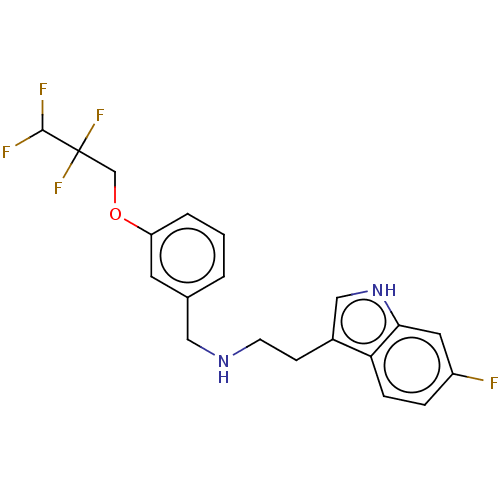
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754

(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592783
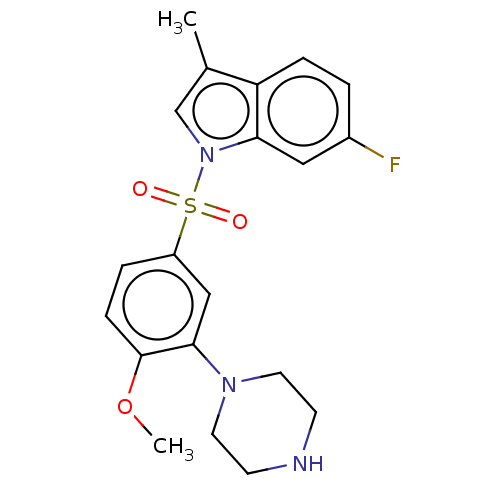
(CHEMBL5201491)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(F)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328410
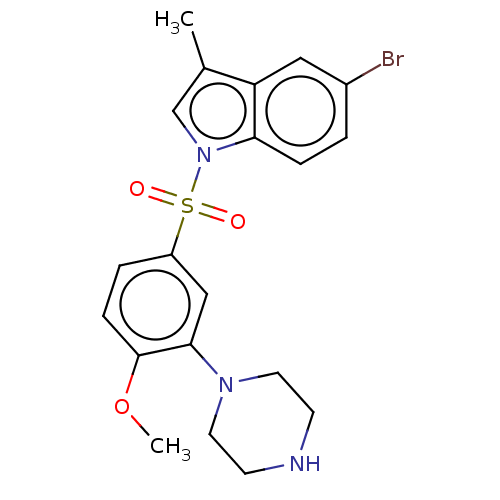
(5-bromo-1-((4-methoxy-3-(piperazin-1-yl)phenyl)sul...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2cc(Br)ccc12 Show InChI InChI=1S/C20H22BrN3O3S/c1-14-13-24(18-5-3-15(21)11-17(14)18)28(25,26)16-4-6-20(27-2)19(12-16)23-9-7-22-8-10-23/h3-6,11-13,22H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286345
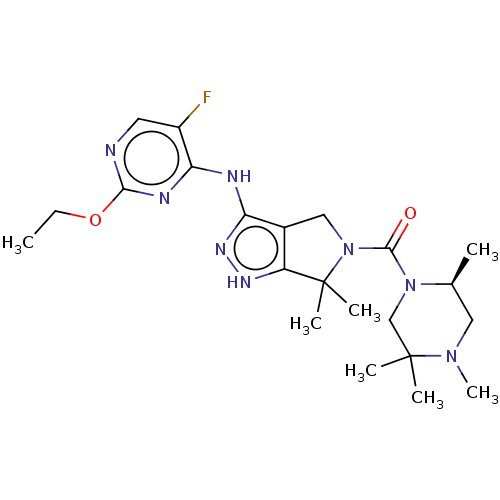
(US11220518, Ex. No. K3 | US11780853, Example K3 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286345

(US11220518, Ex. No. K3 | US11780853, Example K3 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM286345

(US11220518, Ex. No. K3 | US11780853, Example K3 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US9518060 (2016)
BindingDB Entry DOI: 10.7270/Q2ZK5JPN |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286246

(5-{[(8S)-6,8-dimethyl-6,9-diazaspiro[4.5]dec-9-yl]...)Show SMILES C[C@H]1CN(C)C2(CCCC2)CN1C(=O)N1Cc2c(Nc3nc(C)ncc3F)n[nH]c2C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM286246

(5-{[(8S)-6,8-dimethyl-6,9-diazaspiro[4.5]dec-9-yl]...)Show SMILES C[C@H]1CN(C)C2(CCCC2)CN1C(=O)N1Cc2c(Nc3nc(C)ncc3F)n[nH]c2C1(C)C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US9518060 (2016)
BindingDB Entry DOI: 10.7270/Q2ZK5JPN |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286246

(5-{[(8S)-6,8-dimethyl-6,9-diazaspiro[4.5]dec-9-yl]...)Show SMILES C[C@H]1CN(C)C2(CCCC2)CN1C(=O)N1Cc2c(Nc3nc(C)ncc3F)n[nH]c2C1(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM394085

(US9974785, Example 4)Show SMILES FC(F)c1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C19H18BrF2N3O2S/c20-14-4-1-2-7-17(14)28(26,27)25-12-13(19(21)22)18-15(5-3-6-16(18)25)24-10-8-23-9-11-24/h1-7,12,19,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116950
BindingDB Entry DOI: 10.7270/Q2CC14NC |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM533472

(US11220518, Ex. No. K1 | US11780853, Example K1)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM286343

(N-(2-ethoxy-5-fluoropyrimidin-4-yl)-6,6-dimethyl-5...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(C)CC2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US9518060 (2016)
BindingDB Entry DOI: 10.7270/Q2ZK5JPN |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM533472

(US11220518, Ex. No. K1 | US11780853, Example K1)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM533459

(US11220518, Ex. No. J6 | US11780853, Example J6)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(C)ccc3F)n[nH]c2C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM533459

(US11220518, Ex. No. J6 | US11780853, Example J6)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(C)ccc3F)n[nH]c2C1(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM286320
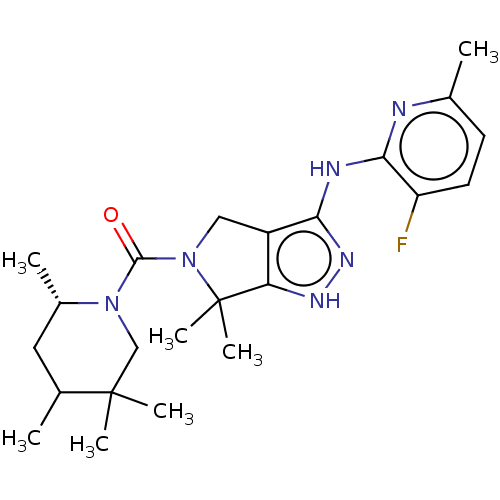
(US9518060, Example J6)Show SMILES C[C@H]1CC(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(C)ccc3F)n[nH]c2C1(C)C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
PFIZER INC.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US9518060 (2016)
BindingDB Entry DOI: 10.7270/Q2ZK5JPN |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50021498
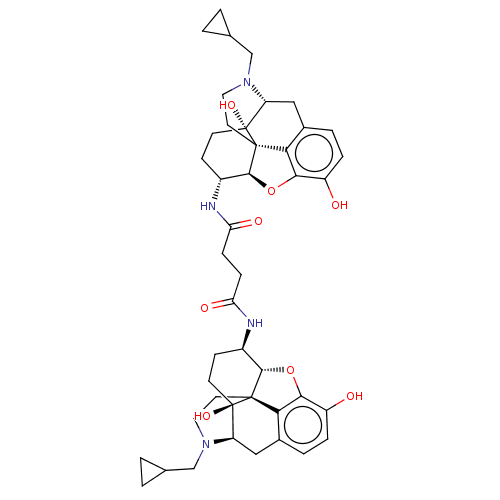
(1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...)Show SMILES [H][C@@]12Oc3c4c(C[C@H]5N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)CCC(=O)N[C@@H]1CC[C@@]2(O)[C@H]4Cc5ccc(O)c6O[C@]1([H])[C@]2(CCN4CC1CC1)c56)ccc3O |TLB:49:48:32:53.36.35,THB:9:8:16:4.5.6| Show InChI InChI=1S/C44H54N4O8/c49-29-7-5-25-19-31-43(53)13-11-27(39-41(43,35(25)37(29)55-39)15-17-47(31)21-23-1-2-23)45-33(51)9-10-34(52)46-28-12-14-44(54)32-20-26-6-8-30(50)38-36(26)42(44,40(28)56-38)16-18-48(32)22-24-3-4-24/h5-8,23-24,27-28,31-32,39-40,49-50,53-54H,1-4,9-22H2,(H,45,51)(H,46,52)/t27-,28-,31?,32?,39+,40+,41?,42?,43-,44-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DADLE from Opioid receptor kappa 1 in guinea pig brain membrane |
J Med Chem 29: 1855-61 (1986)
BindingDB Entry DOI: 10.7270/Q21Z43DQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328377
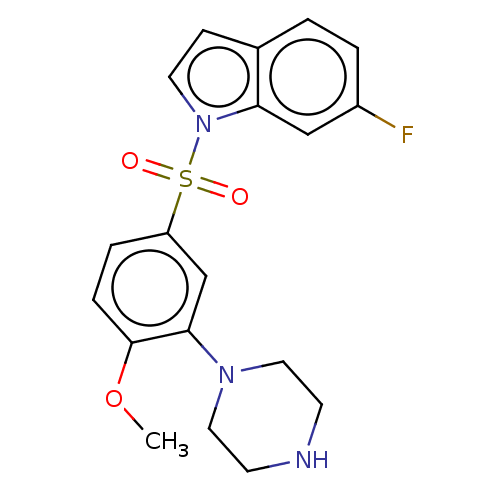
(6-fluoro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(F)cc12 Show InChI InChI=1S/C19H20FN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592786
(CHEMBL5187416)Show SMILES COc1cc(NS(=O)(=O)c2sc3cc(Cl)ccc3c2C)ccc1N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data