

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13152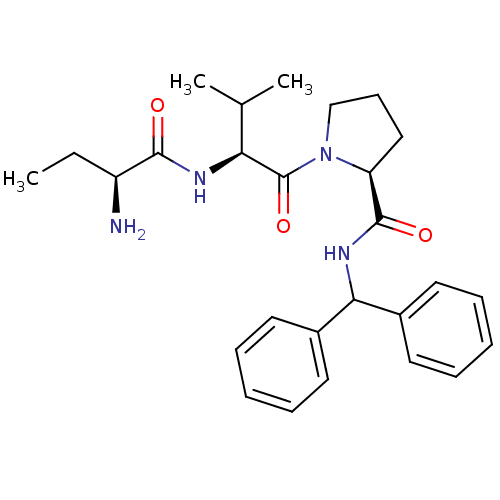 ((2S)-1-[(2S)-2-[(2S)-2-aminobutanamido]-3-methylbu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13151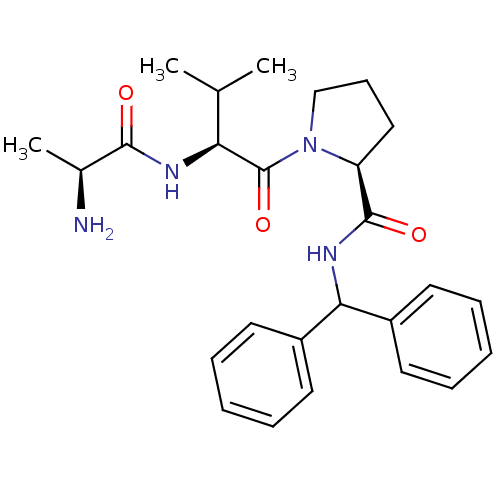 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13155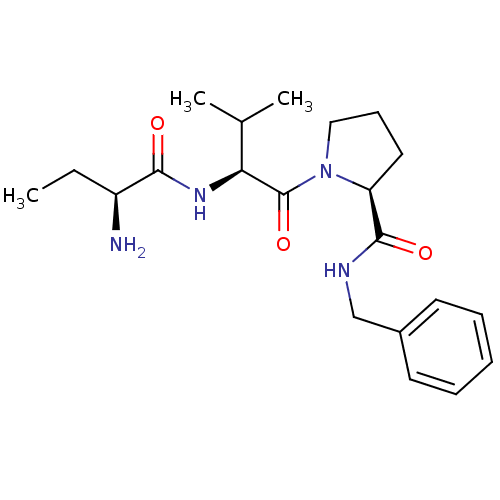 ((2S)-1-[(2S)-2-[(2S)-2-aminobutanamido]-3-methylbu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13150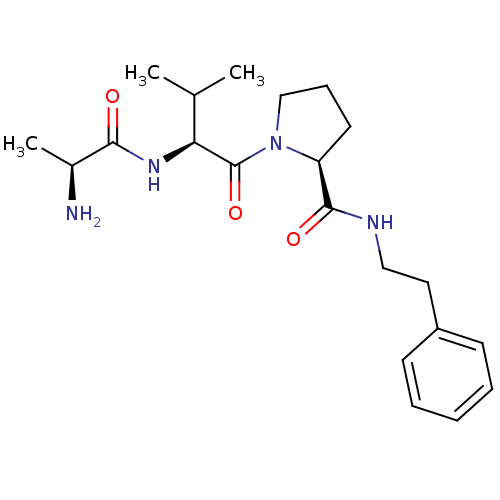 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13148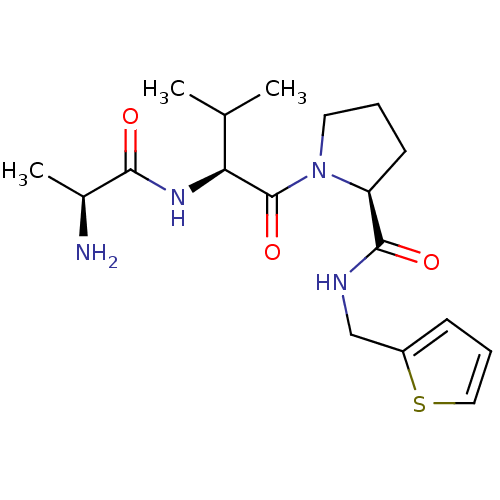 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 180 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13147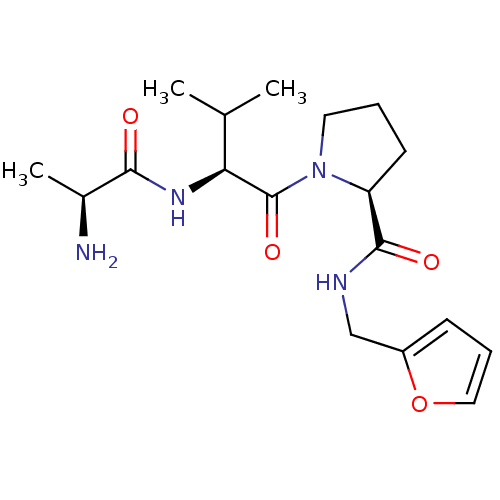 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | -37.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13142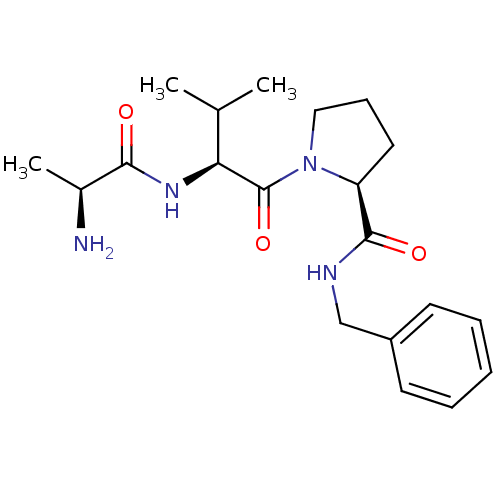 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50437693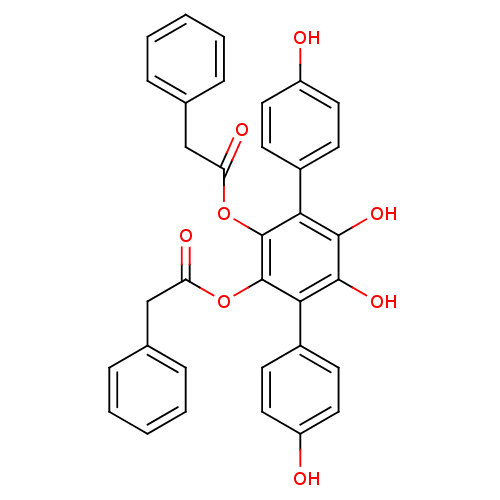 (VIALININ A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Binding affinity to recombinant human 6His-tagged SENP1 catalytic domain expressed in Escherichia coli | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50437693 (VIALININ A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Binding affinity to full-length recombinant human 6His-tagged SENP1 expressed in Escherichia coli BL21 (DE3) | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13153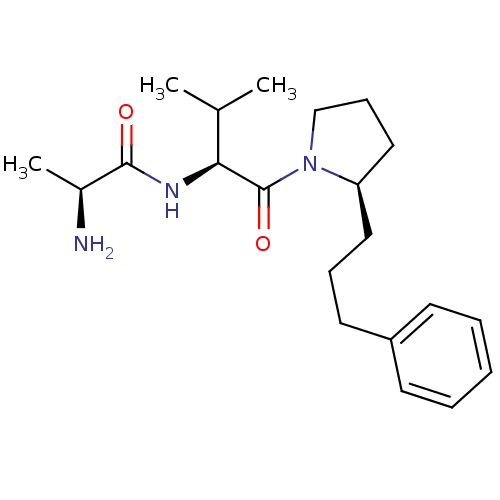 ((2S)-2-amino-N-[(2S)-3-methyl-1-oxo-1-[(2R)-2-(3-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13146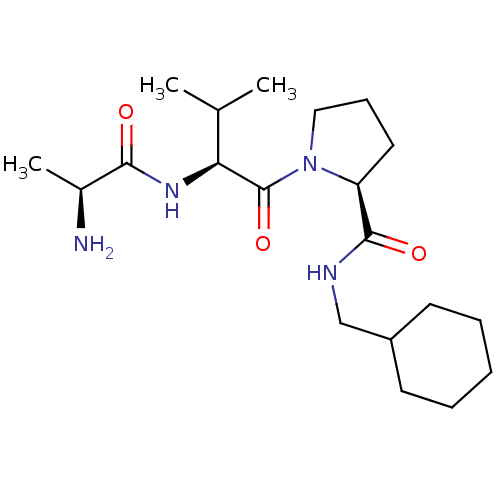 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.27E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 5 (Homo sapiens (Human)) | BDBM50437693 (VIALININ A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of wild type human USP5 expressed in Escherichia coli BL21(DE3) using Ub-AMC as substrate after 60 mins by fluorometric analysis | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13156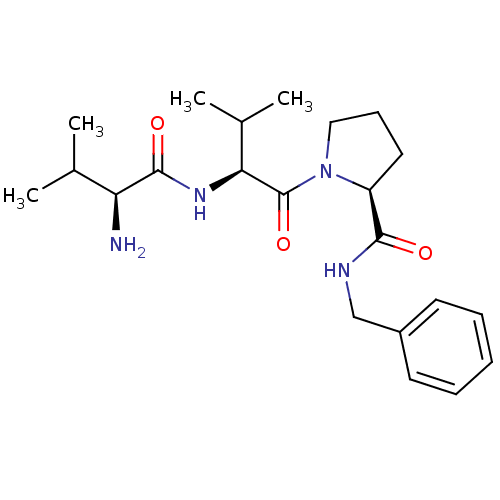 ((2S)-1-[(2S)-2-[(2S)-2-amino-3-methylbutanamido]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.15E+3 | -30.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13145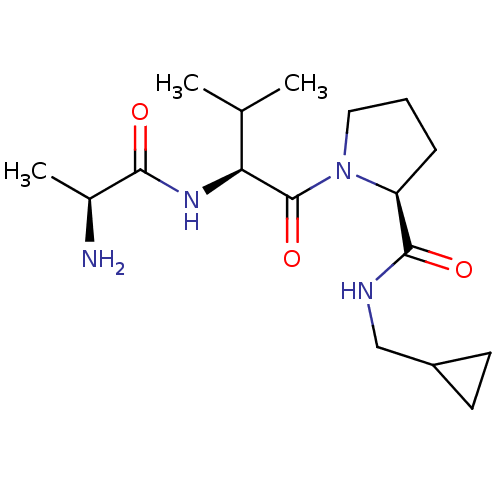 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.41E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13149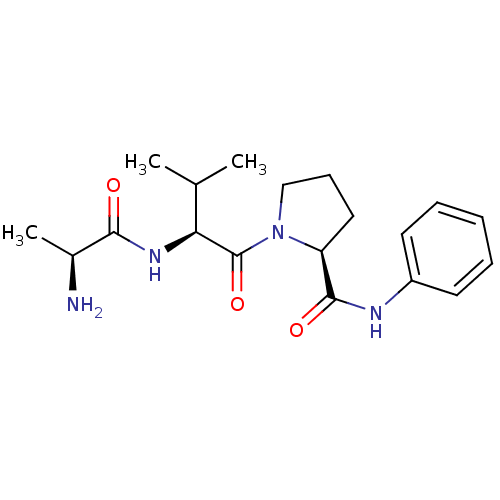 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 5 (Homo sapiens (Human)) | BDBM50437693 (VIALININ A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Competitive inhibition of wild type human USP5 expressed in Escherichia coli BL21(DE3) using Ub-AMC as substrate | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13143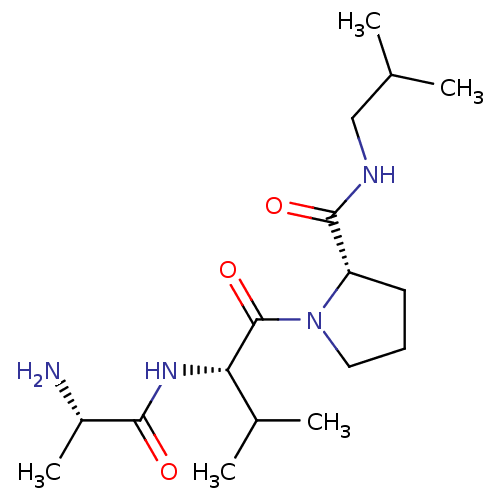 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.34E+4 | -27.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13144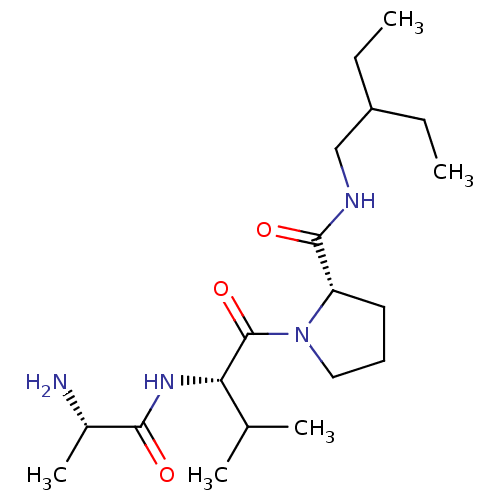 ((2S)-1-[(2S)-2-[(2S)-2-aminopropanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.45E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13157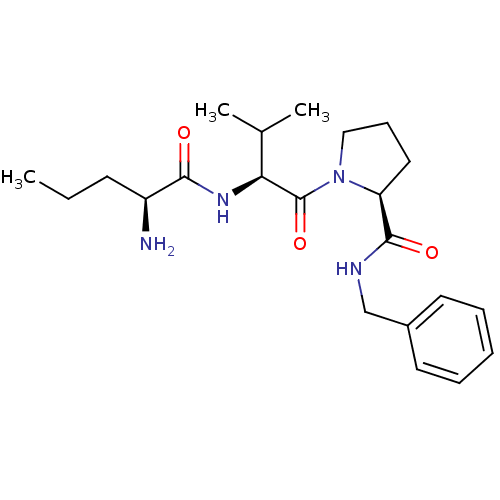 ((2S)-1-[(2S)-2-[(2S)-2-aminopentanamido]-3-methylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.40E+4 | -24.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13154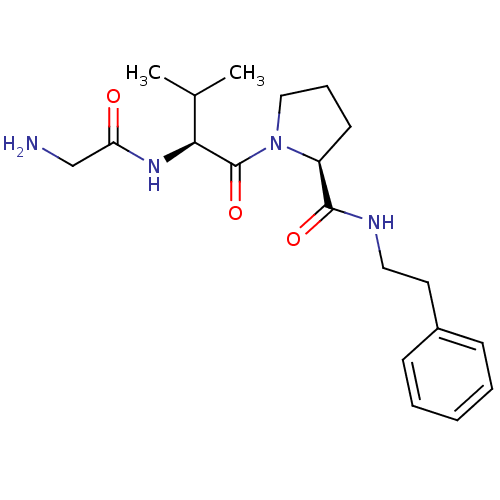 ((2S)-1-[(2S)-2-(2-aminoacetamido)-3-methylbutanoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80E+4 | -23.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Michigan | Assay Description A quantitative in vitro binding assay using the fluorescence polarization (FP) based method was developed and used to determine the binding affinity ... | Bioorg Med Chem Lett 15: 793-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.008 BindingDB Entry DOI: 10.7270/Q2H41PP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005232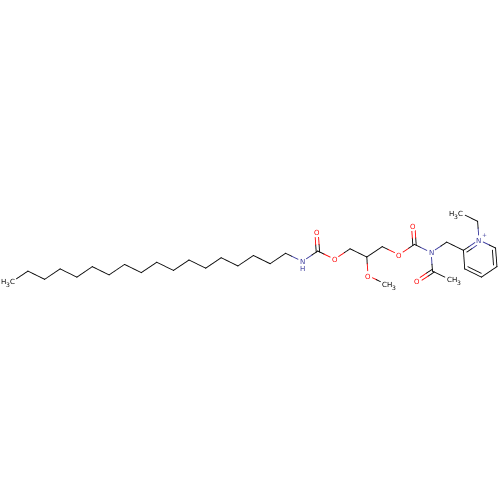 ((R)-2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50367841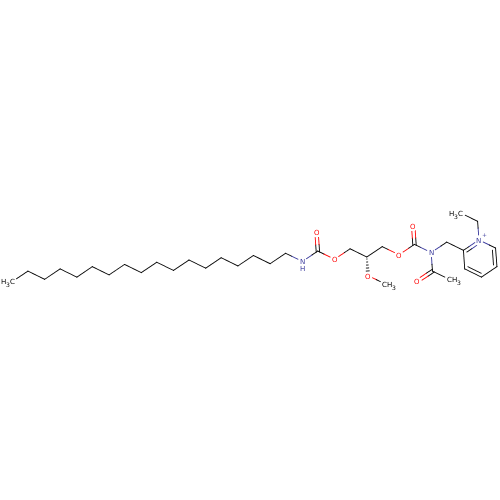 (CHEMBL1788193) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50405738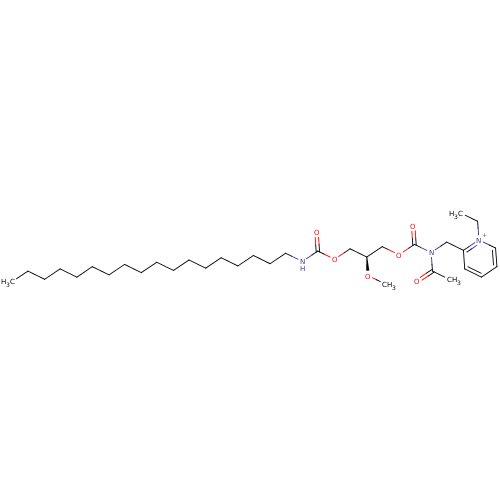 (CHEMBL2093039) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020324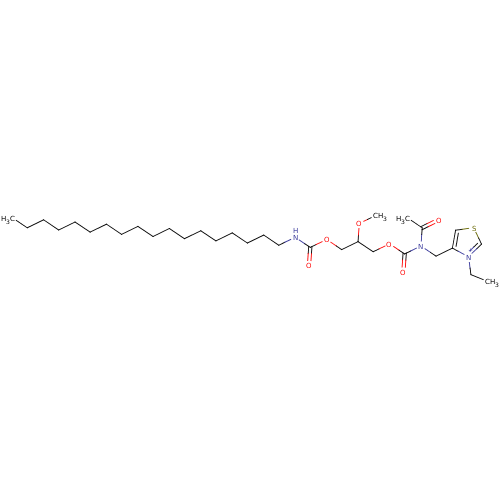 (4-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-prop...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020321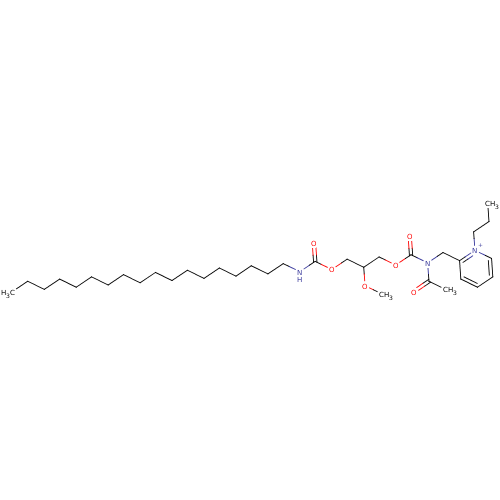 (2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-prop...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020318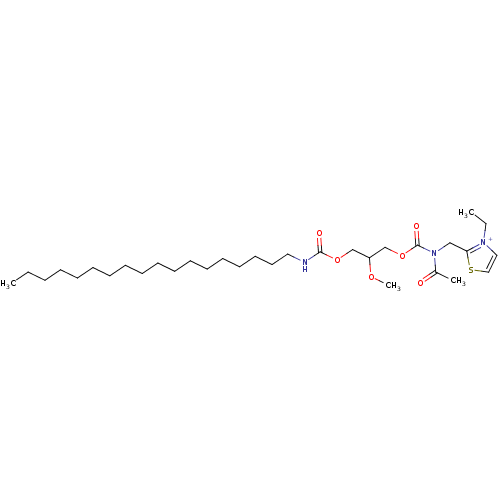 (2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-prop...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020337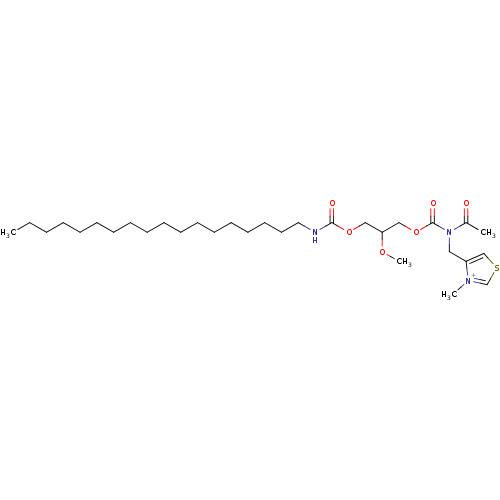 (4-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-prop...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020328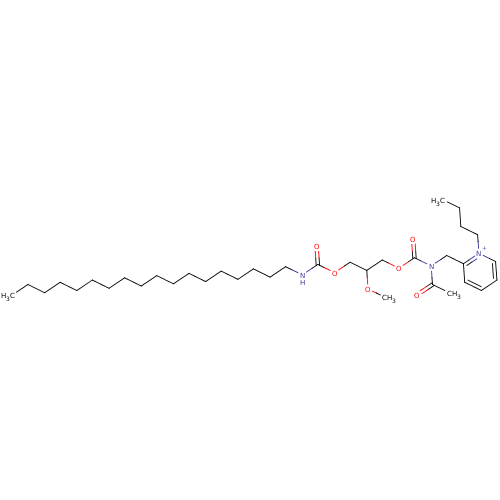 (2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-prop...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020325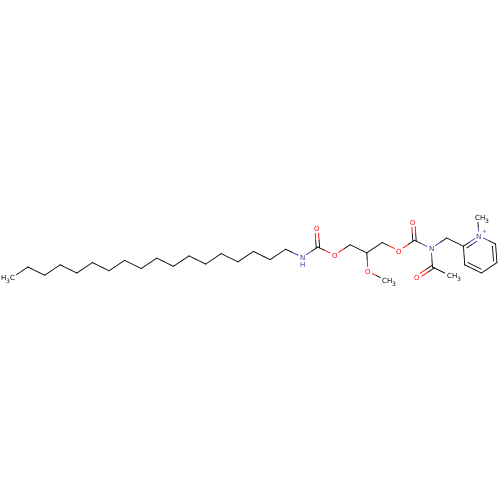 (2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-prop...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020340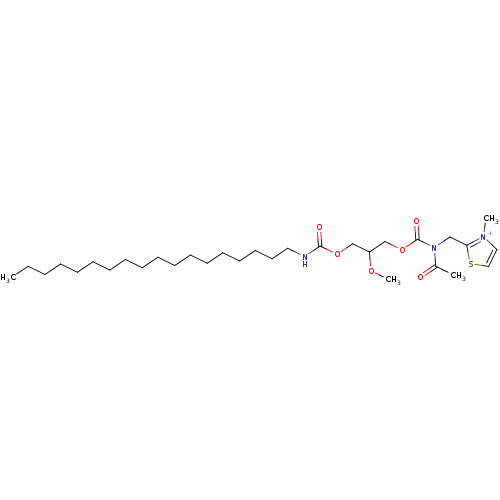 (2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-prop...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020327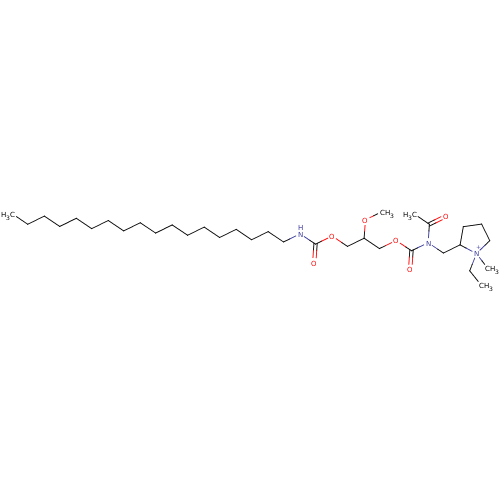 (2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-prop...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020332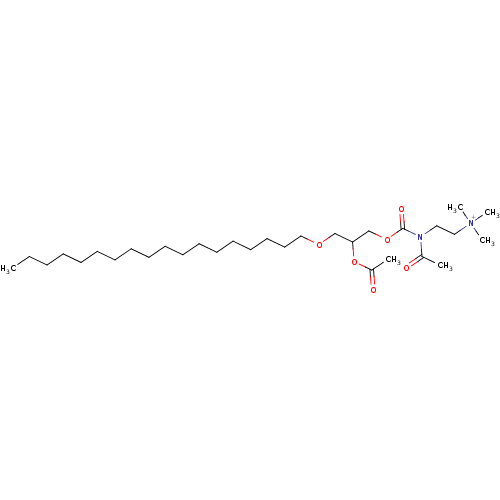 (CHEMBL158254 | {2-[(2-Acetoxy-3-octadecyloxy-propo...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020330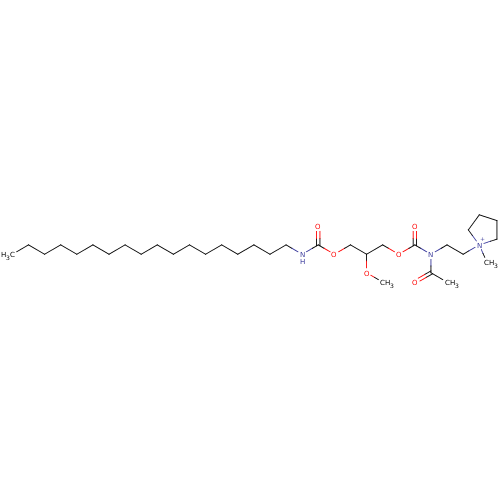 (1-{2-[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-pr...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020329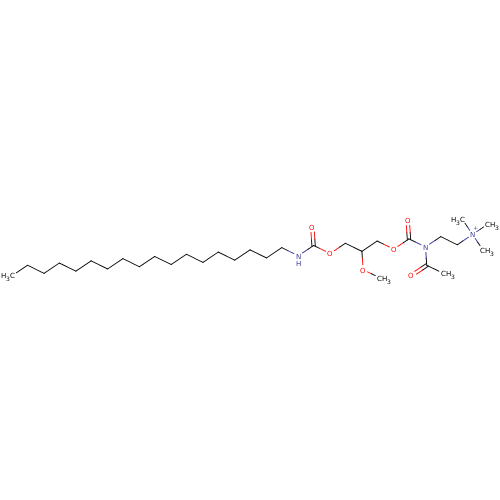 (CHEMBL158489 | {2-[Acetyl-(2-methoxy-3-octadecylca...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 4 (Homo sapiens (Human)) | BDBM50437693 (VIALININ A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of human USP4 using Ub-AMC as substrate after 60 mins by fluorometric analysis | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50536673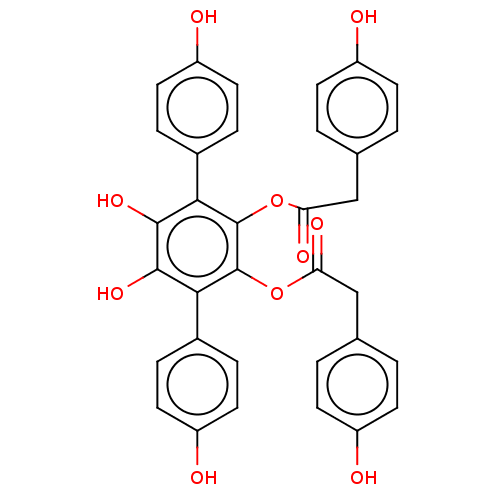 (CHEMBL4587307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged SENP1 catalytic domain expressed in Escherichia coli preincubated for 15 mins followed by addition of SUM... | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50437693 (VIALININ A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human 6His-tagged SENP1 expressed in Escherichia coli BL21 (DE3) preincubated for 15 mins followed by addition ... | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50437693 (VIALININ A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged SENP1 catalytic domain expressed in Escherichia coli preincubated for 15 mins followed by addition of SUM... | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020333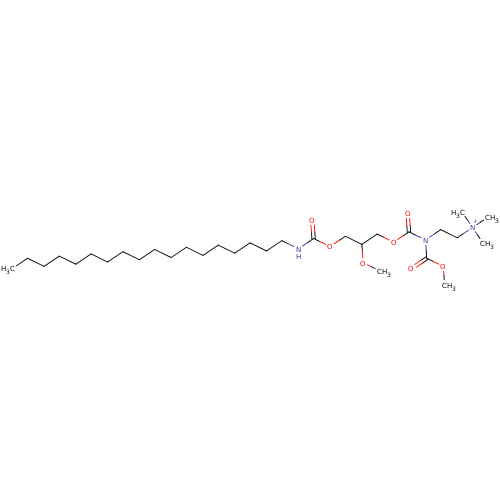 (CHEMBL160653 | {2-[Butyryl-(2-methoxy-3-octadecylc...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50536673 (CHEMBL4587307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human 6His-tagged SENP1 expressed in Escherichia coli BL21 (DE3) preincubated for 15 mins followed by addition ... | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50536672 (CHEMBL2012939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human 6His-tagged SENP1 expressed in Escherichia coli BL21 (DE3) preincubated for 15 mins followed by addition ... | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase isozyme L1 (Homo sapiens (Human)) | BDBM50437695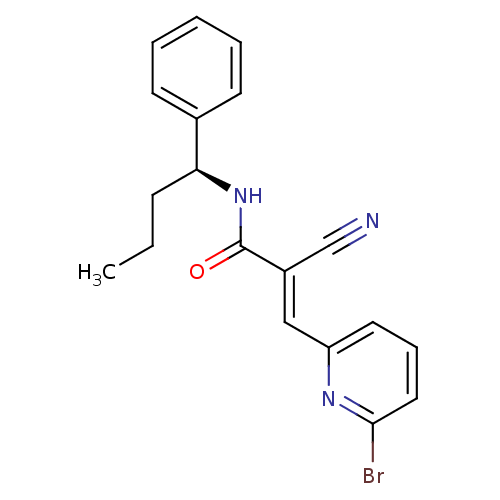 (CHEMBL1923233) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of UCH-L1 in human Z138 cells after 1 hr by immunoblotting analysis | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 5 (Homo sapiens (Human)) | BDBM50437695 (CHEMBL1923233) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of USP5 in human Z138 cells after 1 hr by immunoblotting analysis | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable ubiquitin carboxyl-terminal hydrolase FAF-X (Homo sapiens (Human)) | BDBM50437695 (CHEMBL1923233) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of USP9x in human Z138 cells after 1 hr by immunoblotting analysis | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50536672 (CHEMBL2012939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged SENP1 catalytic domain expressed in Escherichia coli preincubated for 15 mins followed by addition of SUM... | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM50536674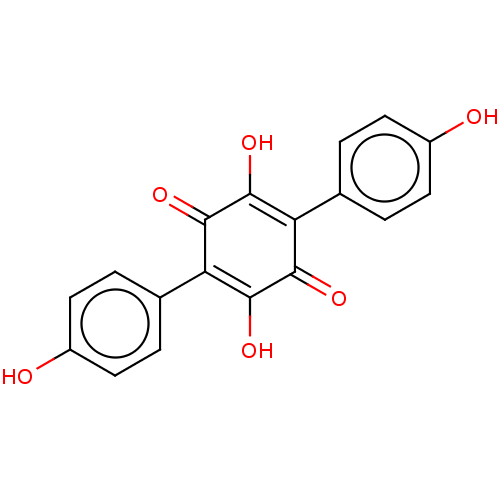 (CHEMBL4593579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human 6His-tagged SENP1 expressed in Escherichia coli BL21 (DE3) preincubated for 15 mins followed by addition ... | Bioorg Med Chem Lett 26: 4237-40 (2016) Article DOI: 10.1016/j.bmcl.2016.07.051 BindingDB Entry DOI: 10.7270/Q2SF30PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 14 (Homo sapiens (Human)) | BDBM50437694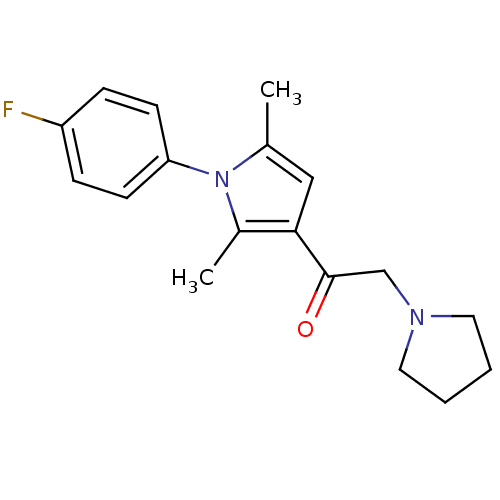 (CHEMBL1410015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture Curated by ChEMBL | Assay Description Inhibition of human USP14 | Bioorg Med Chem Lett 23: 4328-31 (2013) Article DOI: 10.1016/j.bmcl.2013.05.093 BindingDB Entry DOI: 10.7270/Q2T15528 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020319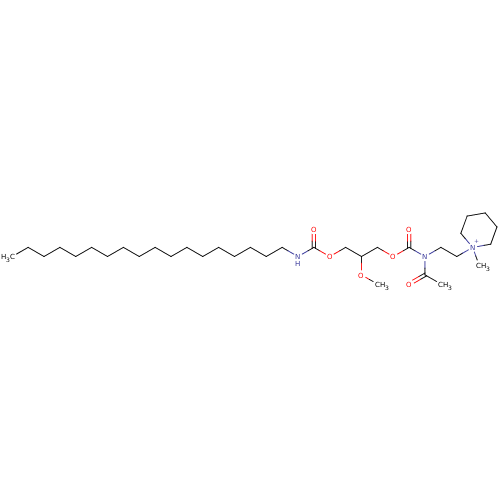 (1-{2-[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-pr...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020320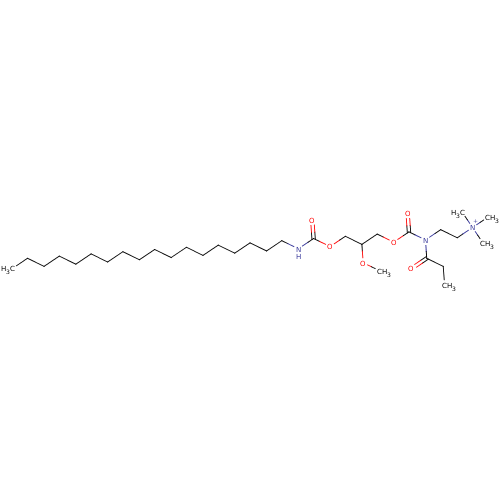 (CHEMBL160910 | {2-[(2-Methoxy-3-octadecylcarbamoyl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50020322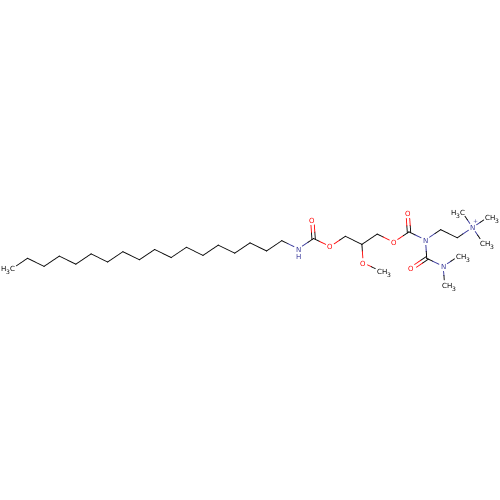 (CHEMBL350437 | {2-[Butyryl-(2-methoxy-3-octadecylc...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit platelet aggregation induced by platelet activating factor (PAF) | J Med Chem 32: 56-64 (1989) BindingDB Entry DOI: 10.7270/Q2ZP46QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |