

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50208446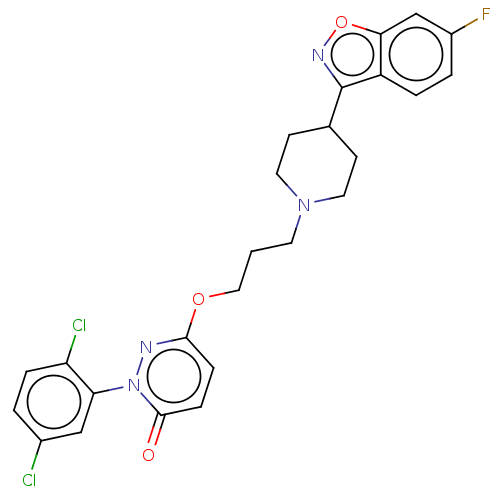 (CHEMBL3884161) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting analy... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50208470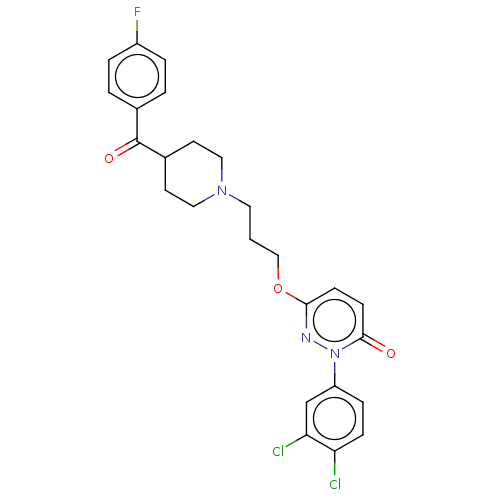 (CHEMBL3885003) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting analy... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta4 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain nicotinic acetylcholine receptor using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta4 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50143281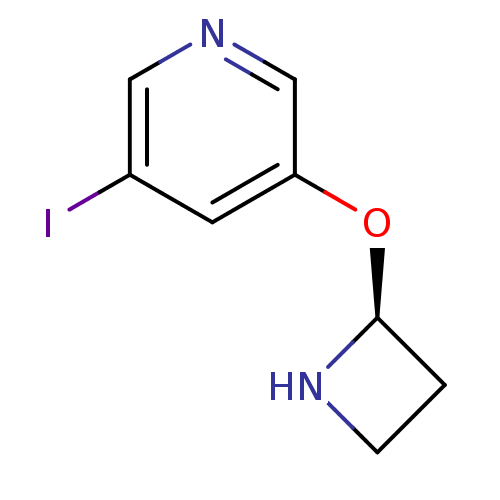 (3-((S)-Azetidin-2-yloxy)-5-iodo-pyridine | CHEMBL5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha3-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to rat Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143281 (3-((S)-Azetidin-2-yloxy)-5-iodo-pyridine | CHEMBL5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta4 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568690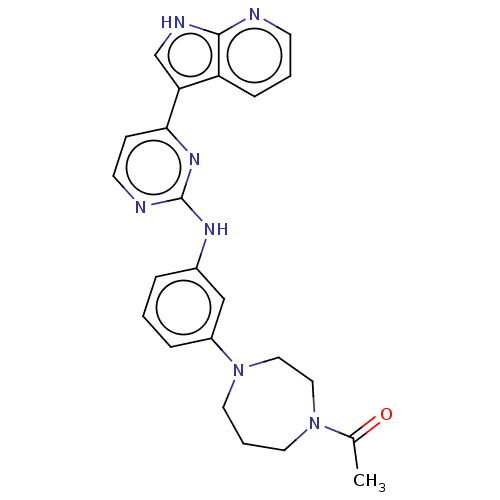 (CHEMBL4870188) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568686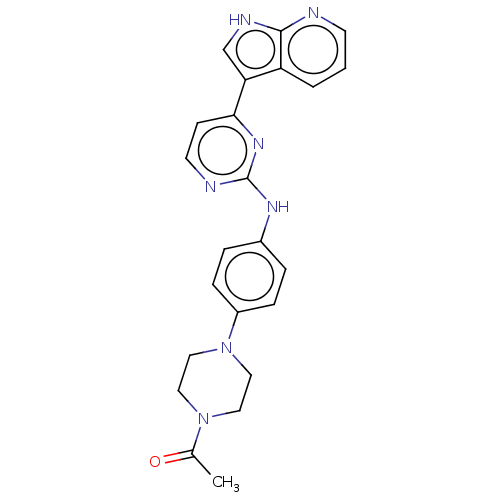 (CHEMBL4864449) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423299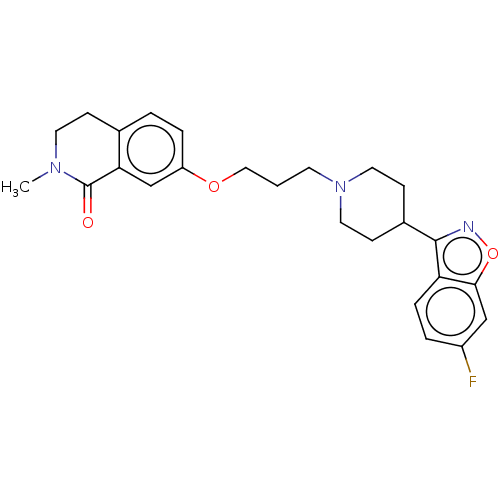 (US10501452, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H] ketanserin from 5-HT2A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting ana... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-ketanserine from serotonin 5-HT2A receptor in rat brain cortex homogenates incubated for 30 mins by liquid scintillation countin... | J Med Chem 61: 10017-10039 (2018) Article DOI: 10.1021/acs.jmedchem.8b01096 BindingDB Entry DOI: 10.7270/Q2NS0XK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568703 (CHEMBL4878159) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50208451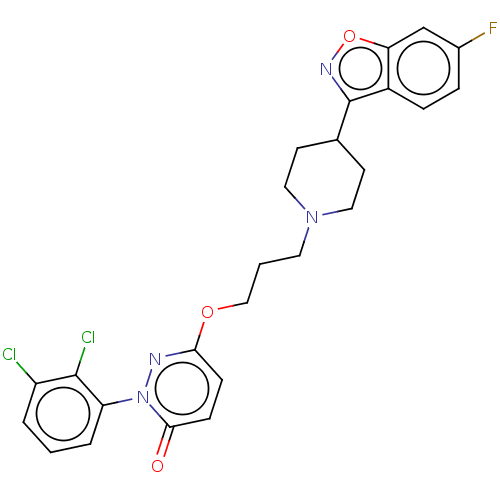 (CHEMBL3885471) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in Sprague-Dawley rat striatum incubated for 30 mins by liquid scintillation counting analysi... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Homo sapiens (Human)) | BDBM50162983 (6-[5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-pyridin-3-yl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha3-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50208447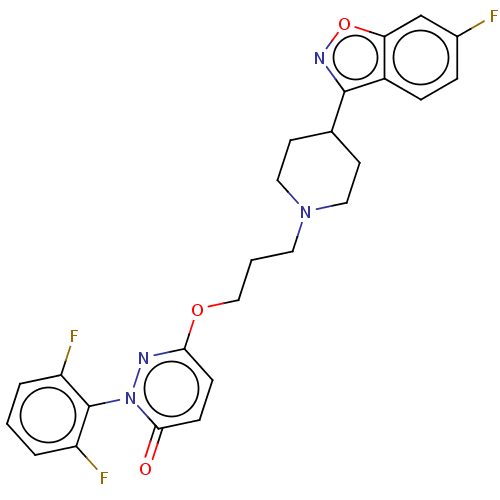 (CHEMBL3883955) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H] ketanserin from 5-HT2A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting ana... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568703 (CHEMBL4878159) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 R595_E596insEY mutant (unknown origin) incubated for 120 mins by ADP-glo based luminescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568703 (CHEMBL4878159) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 D835Y mutant (unknown origin) incubated for 120 mins by ADP-glo based luminescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568703 (CHEMBL4878159) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 F594_R595insR mutant (unknown origin) incubated for 120 mins by ADP-glo based luminescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568698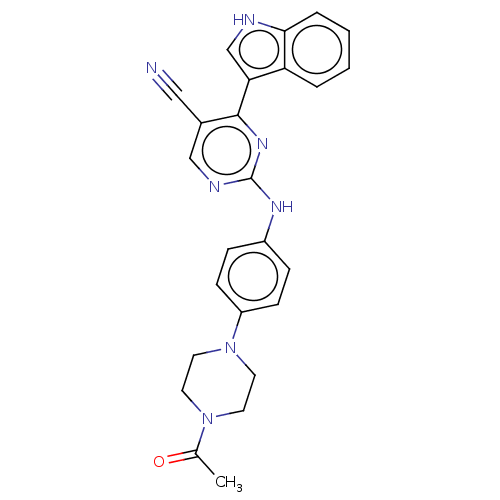 (CHEMBL4855435) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568690 (CHEMBL4870188) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type FLT3 (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50469526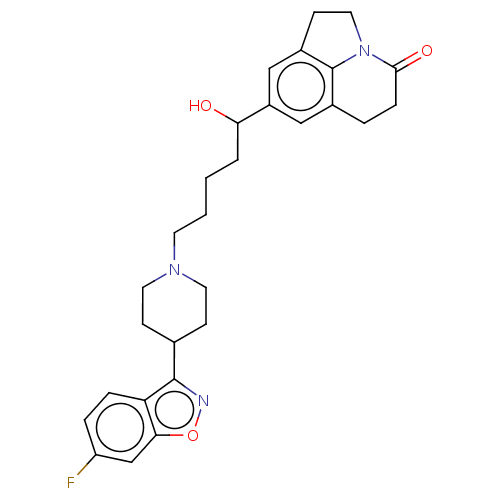 (CHEMBL4290311) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-ketanserine from serotonin 5-HT2A receptor in rat brain cortex homogenates incubated for 30 mins by liquid scintillation countin... | J Med Chem 61: 10017-10039 (2018) Article DOI: 10.1021/acs.jmedchem.8b01096 BindingDB Entry DOI: 10.7270/Q2NS0XK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463294 (CHEMBL4249256) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50208441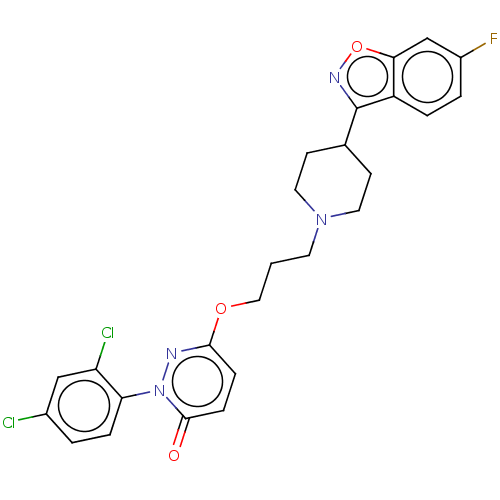 (CHEMBL3885254) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in Sprague-Dawley rat striatum incubated for 30 mins by liquid scintillation counting analysi... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50208441 (CHEMBL3885254) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H] ketanserin from 5-HT2A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting ana... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50143281 (3-((S)-Azetidin-2-yloxy)-5-iodo-pyridine | CHEMBL5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta4 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070780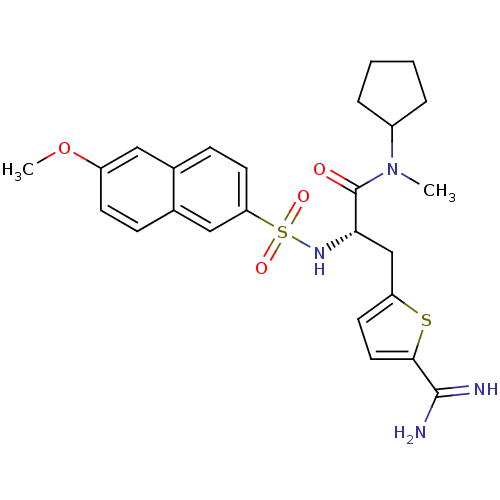 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50208447 (CHEMBL3883955) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in Sprague-Dawley rat striatum incubated for 30 mins by liquid scintillation counting analysi... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50208442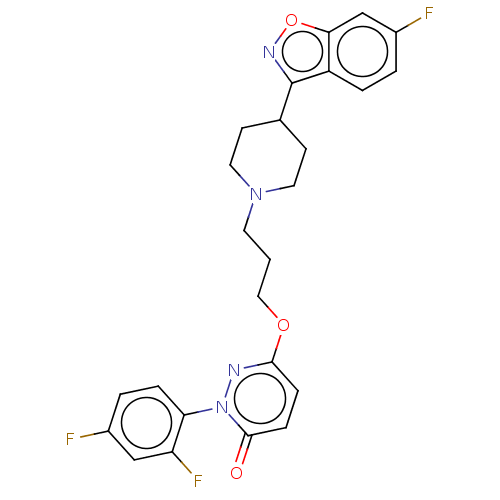 (CHEMBL3884733) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H] ketanserin from 5-HT2A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting ana... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50208451 (CHEMBL3885471) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H] ketanserin from 5-HT2A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting ana... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208447 (CHEMBL3883955) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human recombinant 5-HT6 receptor expressed in CHO cell membranes for 30 mins by liquid scintillat... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50097613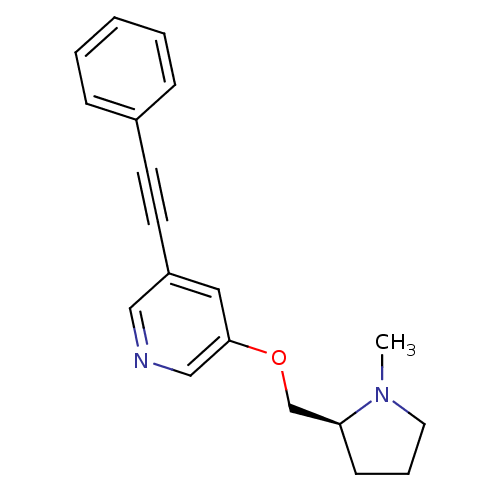 (3-((S)-1-Methyl-pyrrolidin-2-ylmethoxy)-5-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to rat Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to rat Nicotinic acetylcholine receptor alpha3-beta4 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50568704 (CHEMBL4867869) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113215 BindingDB Entry DOI: 10.7270/Q22Z198K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8833 total ) | Next | Last >> |