 Found 937 hits with Last Name = 'yue' and Initial = 'q'
Found 937 hits with Last Name = 'yue' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534504
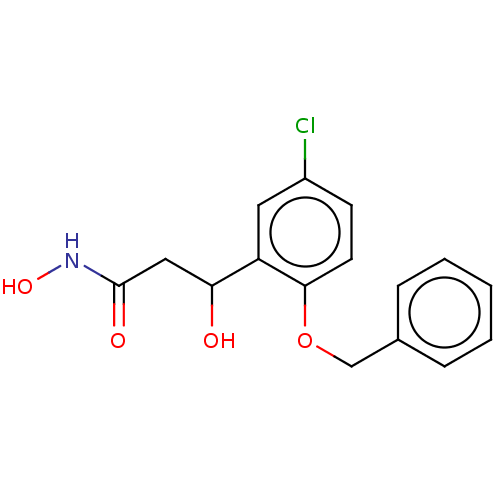
(CHEMBL4590676)Show InChI InChI=1S/C16H16ClNO4/c17-12-6-7-15(22-10-11-4-2-1-3-5-11)13(8-12)14(19)9-16(20)18-21/h1-8,14,19,21H,9-10H2,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-urea-inhibitor complex by non-linear fitt... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534504

(CHEMBL4590676)Show InChI InChI=1S/C16H16ClNO4/c17-12-6-7-15(22-10-11-4-2-1-3-5-11)13(8-12)14(19)9-16(20)18-21/h1-8,14,19,21H,9-10H2,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-inhibitor complex by non-linear fitting a... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493364

(CHEMBL2425480)Show InChI InChI=1S/C9H10ClNO4/c10-5-1-2-7(12)6(3-5)8(13)4-9(14)11-15/h1-3,8,12-13,15H,4H2,(H,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-urea-inhibitor complex by non-linear fitt... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493364

(CHEMBL2425480)Show InChI InChI=1S/C9H10ClNO4/c10-5-1-2-7(12)6(3-5)8(13)4-9(14)11-15/h1-3,8,12-13,15H,4H2,(H,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-inhibitor complex by non-linear fitting a... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394918
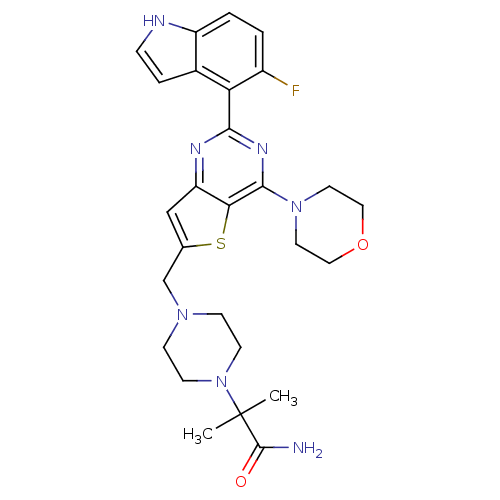
(CHEMBL2165504)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-9-7-33(8-10-35)16-17-15-21-23(38-17)25(34-11-13-37-14-12-34)32-24(31-21)22-18-5-6-30-20(18)4-3-19(22)28/h3-6,15,30H,7-14,16H2,1-2H3,(H2,29,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394917
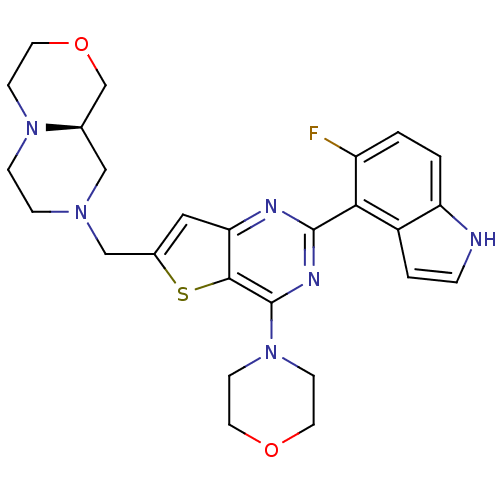
(CHEMBL2165505)Show SMILES Fc1ccc2[nH]ccc2c1-c1nc(N2CCOCC2)c2sc(CN3CCN4CCOC[C@H]4C3)cc2n1 |r| Show InChI InChI=1S/C26H29FN6O2S/c27-20-1-2-21-19(3-4-28-21)23(20)25-29-22-13-18(15-31-5-6-32-7-12-35-16-17(32)14-31)36-24(22)26(30-25)33-8-10-34-11-9-33/h1-4,13,17,28H,5-12,14-16H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM24961
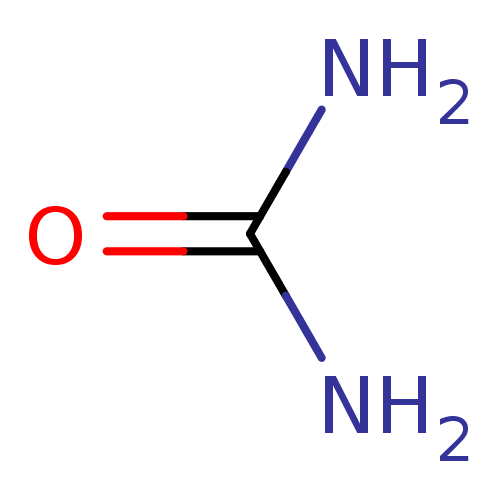
(Urea) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 6.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Substrate inhibition of Helicobacter pylori urease in presence of >4 mM urea by indophenol method |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043407
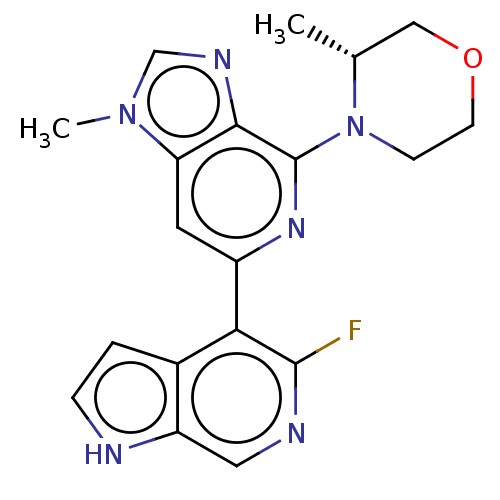
(CHEMBL3355476)Show SMILES C[C@@H]1COCCN1c1nc(cc2n(C)cnc12)-c1c(F)ncc2[nH]ccc12 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ATR using ATP substrate measured after 3 hrs |
ACS Med Chem Lett 6: 42-6 (2015)
Article DOI: 10.1021/ml500352s
BindingDB Entry DOI: 10.7270/Q2XG9SRM |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM202656
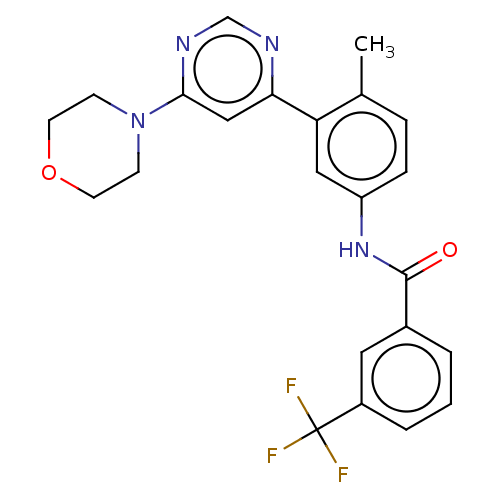
(US10245267, Example 1 | US10709712, Example 1 | US...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cc(ncn1)N1CCOCC1 Show InChI InChI=1S/C23H21F3N4O2/c1-15-5-6-18(29-22(31)16-3-2-4-17(11-16)23(24,25)26)12-19(15)20-13-21(28-14-27-20)30-7-9-32-10-8-30/h2-6,11-14H,7-10H2,1H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043409
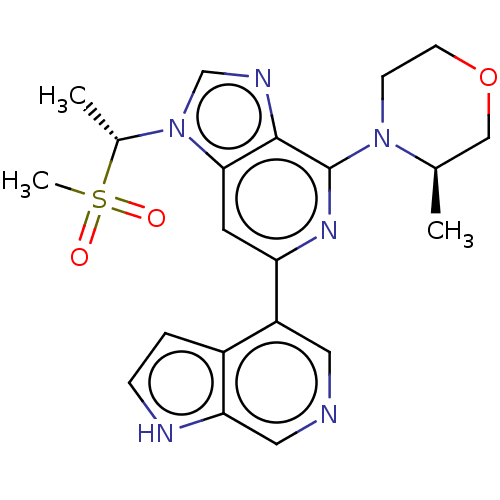
(CHEMBL3355474)Show SMILES C[C@H](n1cnc2c(nc(cc12)-c1cncc2[nH]ccc12)N1CCOC[C@H]1C)S(C)(=O)=O |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ATR using ATP substrate measured after 3 hrs |
ACS Med Chem Lett 6: 42-6 (2015)
Article DOI: 10.1021/ml500352s
BindingDB Entry DOI: 10.7270/Q2XG9SRM |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM88120
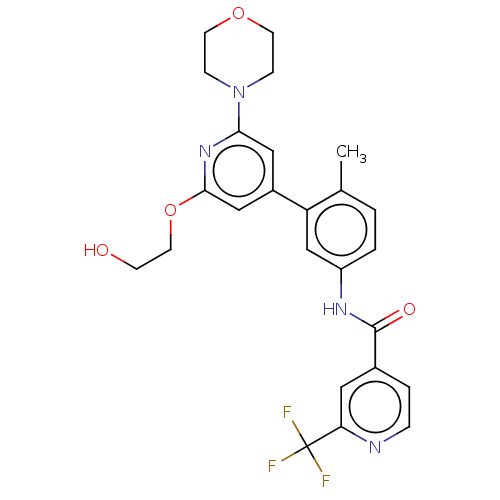
(US10245267, Example 1156 | US10709712, Example 115...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(F)(F)F)cc1-c1cc(OCCO)nc(c1)N1CCOCC1 Show InChI InChI=1S/C25H25F3N4O4/c1-16-2-3-19(30-24(34)17-4-5-29-21(12-17)25(26,27)28)15-20(16)18-13-22(32-6-9-35-10-7-32)31-23(14-18)36-11-8-33/h2-5,12-15,33H,6-11H2,1H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM202686

(US10245267, Example 31 | US10709712, Example 31 | ...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cc(nc(NCCO)n1)N1CCOCC1 Show InChI InChI=1S/C25H26F3N5O3/c1-16-5-6-19(30-23(35)17-3-2-4-18(13-17)25(26,27)28)14-20(16)21-15-22(33-8-11-36-12-9-33)32-24(31-21)29-7-10-34/h2-6,13-15,34H,7-12H2,1H3,(H,30,35)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043385
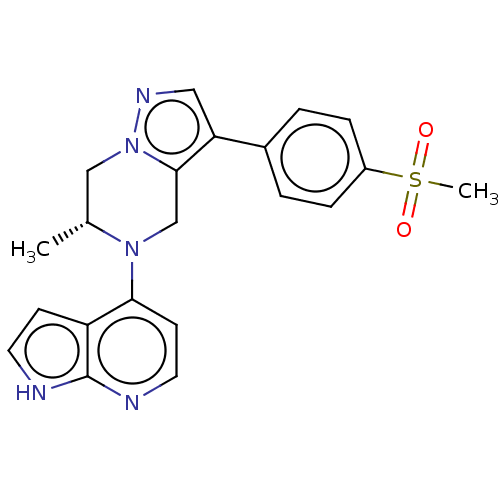
(CHEMBL3355072)Show SMILES C[C@@H]1Cn2ncc(c2CN1c1ccnc2[nH]ccc12)-c1ccc(cc1)S(C)(=O)=O |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay |
ACS Med Chem Lett 6: 37-41 (2015)
Article DOI: 10.1021/ml500353p
BindingDB Entry DOI: 10.7270/Q228097V |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM87998

(US10245267, Example 1029 | US10709712, Example 103...)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cnc(O[C@H]2CCOC[C@H]2F)c(c1)N1CCOCC1 |r| Show InChI InChI=1S/C28H28F4N4O4/c1-17-22(13-21(15-33-17)35-26(37)18-3-2-4-20(11-18)28(30,31)32)19-12-24(36-6-9-38-10-7-36)27(34-14-19)40-25-5-8-39-16-23(25)29/h2-4,11-15,23,25H,5-10,16H2,1H3,(H,35,37)/t23-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM88120

(US10245267, Example 1156 | US10709712, Example 115...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(F)(F)F)cc1-c1cc(OCCO)nc(c1)N1CCOCC1 Show InChI InChI=1S/C25H25F3N4O4/c1-16-2-3-19(30-24(34)17-4-5-29-21(12-17)25(26,27)28)15-20(16)18-13-22(32-6-9-35-10-7-32)31-23(14-18)36-11-8-33/h2-5,12-15,33H,6-11H2,1H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full-length BRAF (unknown origin) |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM202784

(US10245267, Example 131 | US10709712, Example 131 ...)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cnc(OC2CCOCC2)c(c1)N1CCOCC1 Show InChI InChI=1S/C28H29F3N4O4/c1-18-24(15-22(17-32-18)34-26(36)19-3-2-4-21(13-19)28(29,30)31)20-14-25(35-7-11-38-12-8-35)27(33-16-20)39-23-5-9-37-10-6-23/h2-4,13-17,23H,5-12H2,1H3,(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043408
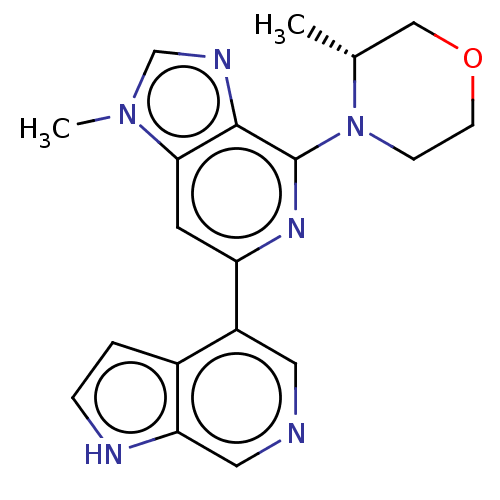
(CHEMBL3355475)Show SMILES C[C@@H]1COCCN1c1nc(cc2n(C)cnc12)-c1cncc2[nH]ccc12 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ATR using ATP substrate measured after 3 hrs |
ACS Med Chem Lett 6: 42-6 (2015)
Article DOI: 10.1021/ml500352s
BindingDB Entry DOI: 10.7270/Q2XG9SRM |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM84365

(US10245267, Example 636 | US10709712, Example 636 ...)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cc(N2CCOCC2)c(OC2CCOCC2)nn1 Show InChI InChI=1S/C27H28F3N5O4/c1-17-22(14-20(16-31-17)32-25(36)18-3-2-4-19(13-18)27(28,29)30)23-15-24(35-7-11-38-12-8-35)26(34-33-23)39-21-5-9-37-10-6-21/h2-4,13-16,21H,5-12H2,1H3,(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043387
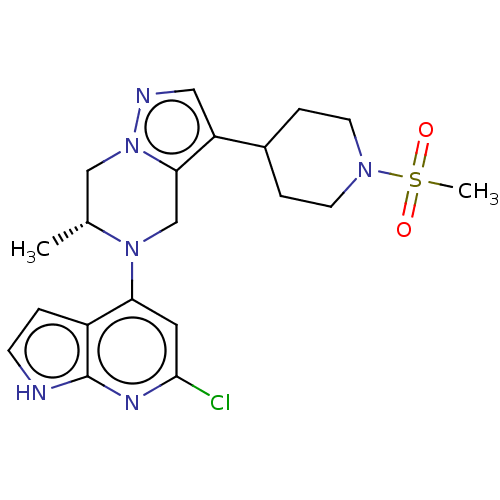
(CHEMBL3355074)Show SMILES C[C@@H]1Cn2ncc(C3CCN(CC3)S(C)(=O)=O)c2CN1c1cc(Cl)nc2[nH]ccc12 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay |
ACS Med Chem Lett 6: 37-41 (2015)
Article DOI: 10.1021/ml500353p
BindingDB Entry DOI: 10.7270/Q228097V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394916
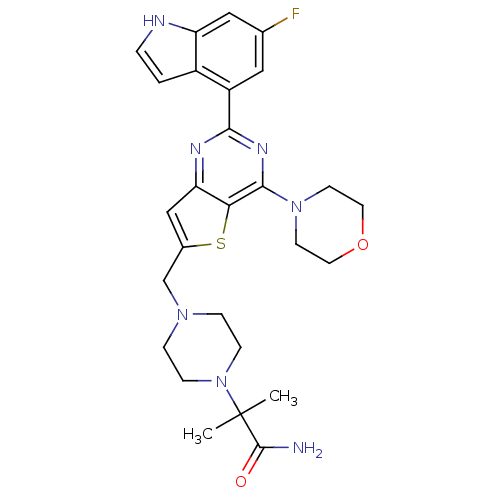
(CHEMBL2165506)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cc(F)cc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-7-5-33(6-8-35)16-18-15-22-23(38-18)25(34-9-11-37-12-10-34)32-24(31-22)20-13-17(28)14-21-19(20)3-4-30-21/h3-4,13-15,30H,5-12,16H2,1-2H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043411

(CHEMBL3355480)Show SMILES C[C@@H]1COCCN1c1nc(cc2n(C)cnc12)-c1nncc2[nH]ccc12 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ATR using ATP substrate measured after 3 hrs |
ACS Med Chem Lett 6: 42-6 (2015)
Article DOI: 10.1021/ml500352s
BindingDB Entry DOI: 10.7270/Q2XG9SRM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043384
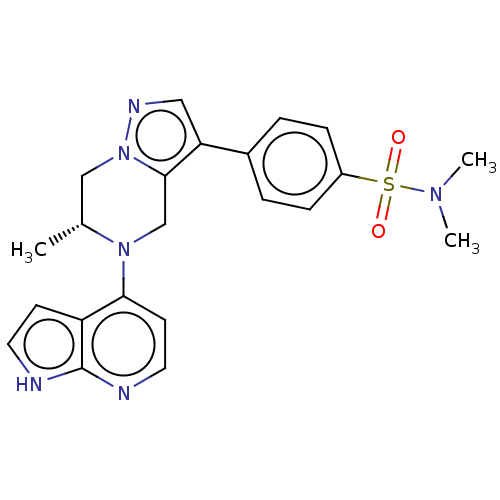
(CHEMBL3355071)Show SMILES C[C@@H]1Cn2ncc(c2CN1c1ccnc2[nH]ccc12)-c1ccc(cc1)S(=O)(=O)N(C)C |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay |
ACS Med Chem Lett 6: 37-41 (2015)
Article DOI: 10.1021/ml500353p
BindingDB Entry DOI: 10.7270/Q228097V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043382

(CHEMBL3355069)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-c1cnc2ccc(nn12)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H18N6O2S/c1-26(2)30(28,29)15-5-3-14(4-6-15)19-13-24-20-8-7-18(25-27(19)20)16-9-11-22-21-17(16)10-12-23-21/h3-13H,1-2H3,(H,22,23) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay |
ACS Med Chem Lett 6: 37-41 (2015)
Article DOI: 10.1021/ml500353p
BindingDB Entry DOI: 10.7270/Q228097V |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50510891
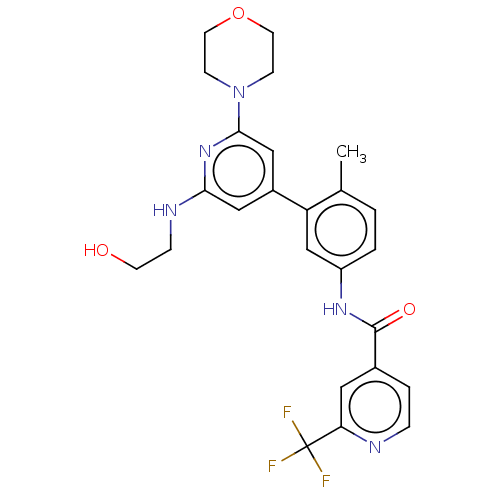
(CHEMBL4455996)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(F)(F)F)cc1-c1cc(NCCO)nc(c1)N1CCOCC1 Show InChI InChI=1S/C25H26F3N5O3/c1-16-2-3-19(31-24(35)17-4-5-29-21(12-17)25(26,27)28)15-20(16)18-13-22(30-6-9-34)32-23(14-18)33-7-10-36-11-8-33/h2-5,12-15,34H,6-11H2,1H3,(H,30,32)(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394910
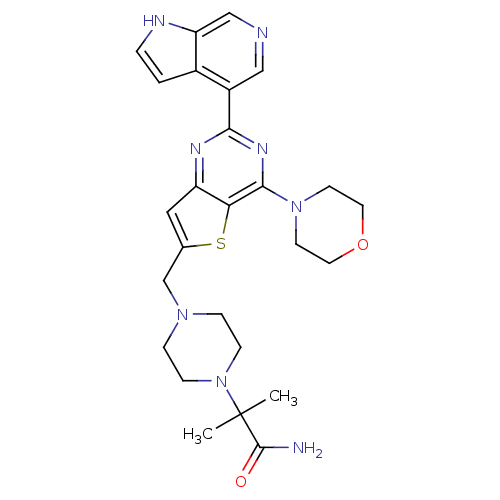
(CHEMBL2165512)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cncc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-7-5-32(6-8-34)16-17-13-20-22(37-17)24(33-9-11-36-12-10-33)31-23(30-20)19-14-28-15-21-18(19)3-4-29-21/h3-4,13-15,29H,5-12,16H2,1-2H3,(H2,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM88006
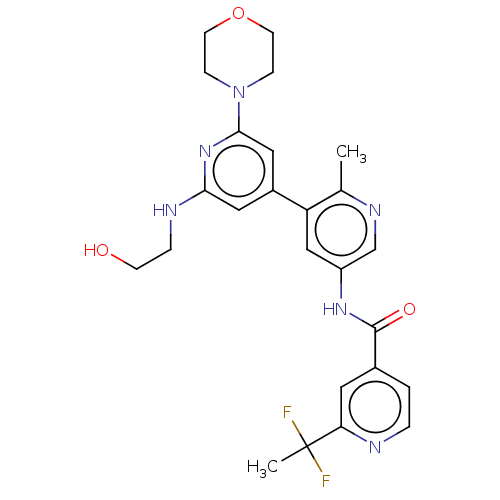
(US10245267, Example 1041 | US10709712, Example 104...)Show SMILES Cc1ncc(NC(=O)c2ccnc(c2)C(C)(F)F)cc1-c1cc(NCCO)nc(c1)N1CCOCC1 Show InChI InChI=1S/C25H28F2N6O3/c1-16-20(18-12-22(29-5-8-34)32-23(13-18)33-6-9-36-10-7-33)14-19(15-30-16)31-24(35)17-3-4-28-21(11-17)25(2,26)27/h3-4,11-15,34H,5-10H2,1-2H3,(H,29,32)(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50510890
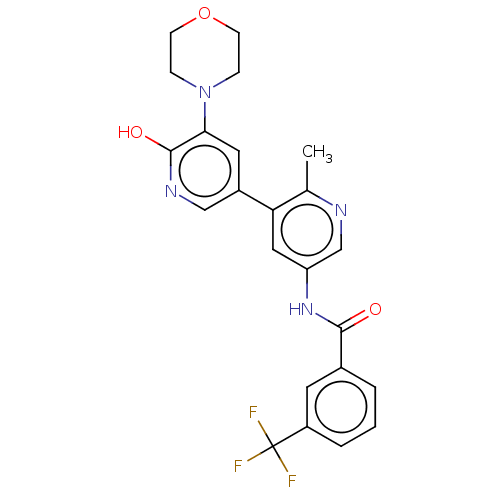
(CHEMBL4593446)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cnc(O)c(c1)N1CCOCC1 Show InChI InChI=1S/C23H21F3N4O3/c1-14-19(16-10-20(22(32)28-12-16)30-5-7-33-8-6-30)11-18(13-27-14)29-21(31)15-3-2-4-17(9-15)23(24,25)26/h2-4,9-13H,5-8H2,1H3,(H,28,32)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597908

(CHEMBL5181496)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(NC2CCCCC2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597908

(CHEMBL5181496)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(NC2CCCCC2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043410

(CHEMBL3355473)Show SMILES C[C@@H]1COCCN1c1nc(cc2n(CS(C)(=O)=O)cnc12)-c1cccc2[nH]ccc12 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ATR using ATP substrate measured after 3 hrs |
ACS Med Chem Lett 6: 42-6 (2015)
Article DOI: 10.1021/ml500352s
BindingDB Entry DOI: 10.7270/Q2XG9SRM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043383
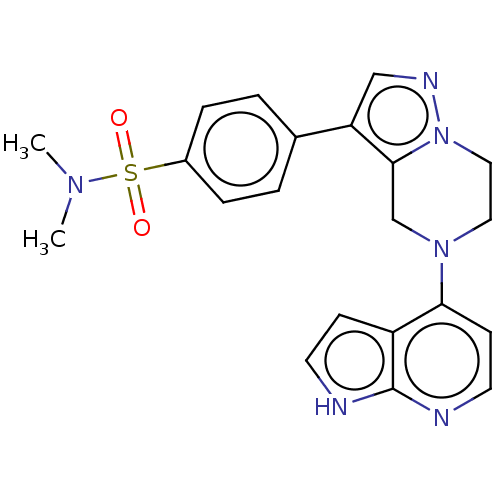
(CHEMBL3355070)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-c1cnn2CCN(Cc12)c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H22N6O2S/c1-25(2)30(28,29)16-5-3-15(4-6-16)18-13-24-27-12-11-26(14-20(18)27)19-8-10-23-21-17(19)7-9-22-21/h3-10,13H,11-12,14H2,1-2H3,(H,22,23) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay |
ACS Med Chem Lett 6: 37-41 (2015)
Article DOI: 10.1021/ml500353p
BindingDB Entry DOI: 10.7270/Q228097V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043386

(CHEMBL3355073)Show SMILES C[C@@H]1Cn2ncc(C3CCN(CC3)S(C)(=O)=O)c2CN1c1ccnc2[nH]ccc12 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay |
ACS Med Chem Lett 6: 37-41 (2015)
Article DOI: 10.1021/ml500353p
BindingDB Entry DOI: 10.7270/Q228097V |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM202839

(US10245267, Example 640 | US10709712, Example 191 ...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(C)(C)C#N)cc1-c1cc(N2CCOCC2)c(=O)n(C)n1 Show InChI InChI=1S/C26H28N6O3/c1-17-5-6-19(29-24(33)18-7-8-28-23(13-18)26(2,3)16-27)14-20(17)21-15-22(25(34)31(4)30-21)32-9-11-35-12-10-32/h5-8,13-15H,9-12H2,1-4H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of alpha-4-beta-2 nAChR |
Bioorg Med Chem Lett 17: 6245-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.026
BindingDB Entry DOI: 10.7270/Q24J0GBN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394913

(CHEMBL2165509)Show SMILES Cc1cc2c(cccc2[nH]1)-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)C(C)(C)C(N)=O)cc2n1 Show InChI InChI=1S/C28H35N7O2S/c1-18-15-21-20(5-4-6-22(21)30-18)25-31-23-16-19(38-24(23)26(32-25)34-11-13-37-14-12-34)17-33-7-9-35(10-8-33)28(2,3)27(29)36/h4-6,15-16,30H,7-14,17H2,1-3H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597906
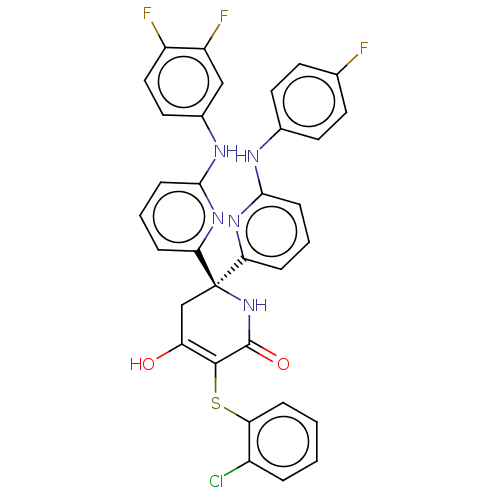
(CHEMBL5183539)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)N[C@](C1)(c1cccc(Nc2ccc(F)cc2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |r,c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50510889
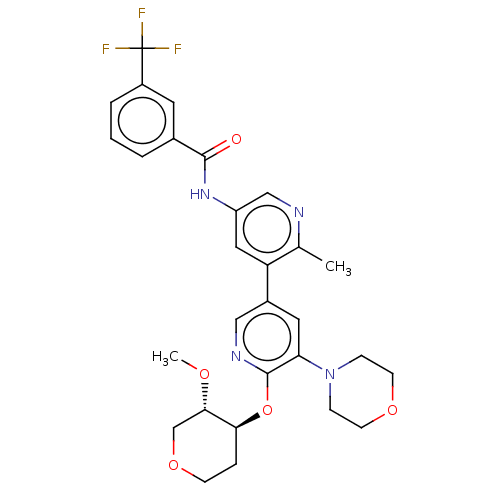
(CHEMBL4475855)Show SMILES CO[C@H]1COCC[C@@H]1Oc1ncc(cc1N1CCOCC1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)cnc1C |r| Show InChI InChI=1S/C29H31F3N4O5/c1-18-23(14-22(16-33-18)35-27(37)19-4-3-5-21(12-19)29(30,31)32)20-13-24(36-7-10-39-11-8-36)28(34-15-20)41-25-6-9-40-17-26(25)38-2/h3-5,12-16,25-26H,6-11,17H2,1-2H3,(H,35,37)/t25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394920
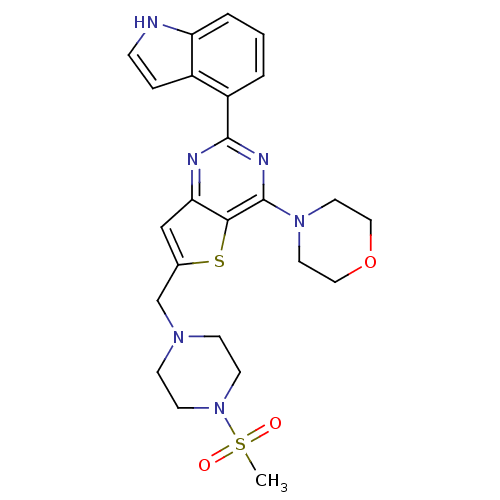
(CHEMBL2165672)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C24H28N6O3S2/c1-35(31,32)30-9-7-28(8-10-30)16-17-15-21-22(34-17)24(29-11-13-33-14-12-29)27-23(26-21)19-3-2-4-20-18(19)5-6-25-20/h2-6,15,25H,7-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597915

(CHEMBL5185237)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Nc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597915

(CHEMBL5185237)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Nc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597916

(CHEMBL5201115)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Oc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597916

(CHEMBL5201115)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Oc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394908
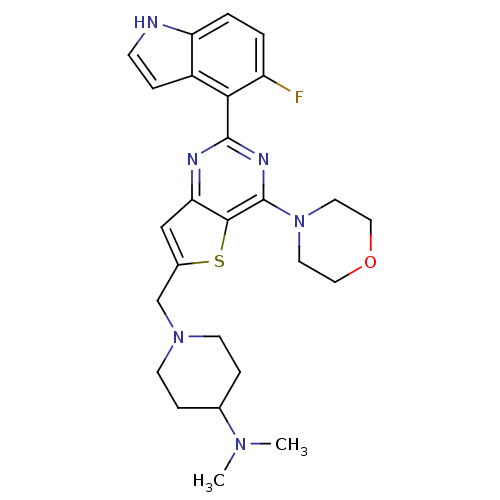
(CHEMBL2165666)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C26H31FN6OS/c1-31(2)17-6-9-32(10-7-17)16-18-15-22-24(35-18)26(33-11-13-34-14-12-33)30-25(29-22)23-19-5-8-28-21(19)4-3-20(23)27/h3-5,8,15,17,28H,6-7,9-14,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394921
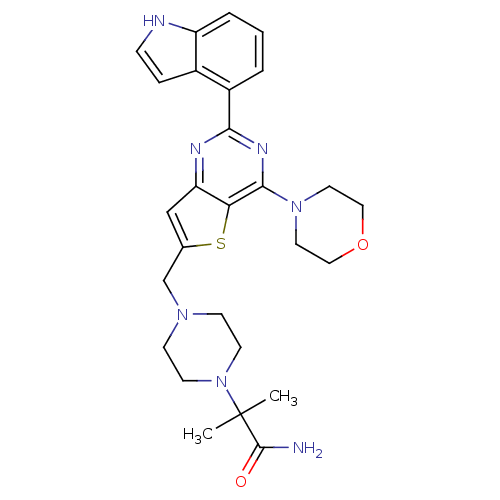
(CHEMBL2165671)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H33N7O2S/c1-27(2,26(28)35)34-10-8-32(9-11-34)17-18-16-22-23(37-18)25(33-12-14-36-15-13-33)31-24(30-22)20-4-3-5-21-19(20)6-7-29-21/h3-7,16,29H,8-15,17H2,1-2H3,(H2,28,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50043389

(CHEMBL3352844)Show SMILES C[C@@H]1Cn2ncc(C3CCN(CC3)S(C)(=O)=O)c2CN1c1ccnc2[nH]cnc12 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay |
ACS Med Chem Lett 6: 37-41 (2015)
Article DOI: 10.1021/ml500353p
BindingDB Entry DOI: 10.7270/Q228097V |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597916

(CHEMBL5201115)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Oc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597896
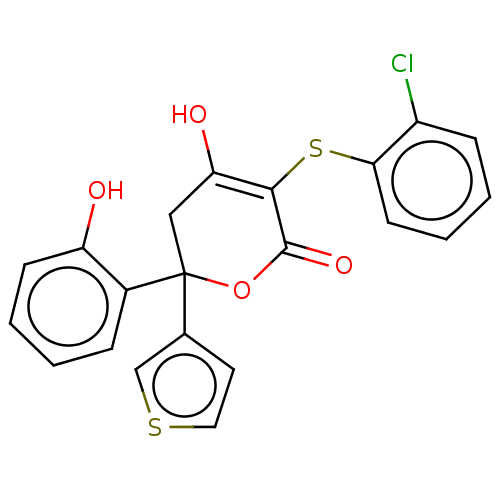
(CHEMBL5181996)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)OC(C1)(c1ccsc1)c1ccccc1O |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597896

(CHEMBL5181996)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)OC(C1)(c1ccsc1)c1ccccc1O |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597916

(CHEMBL5201115)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Oc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394909
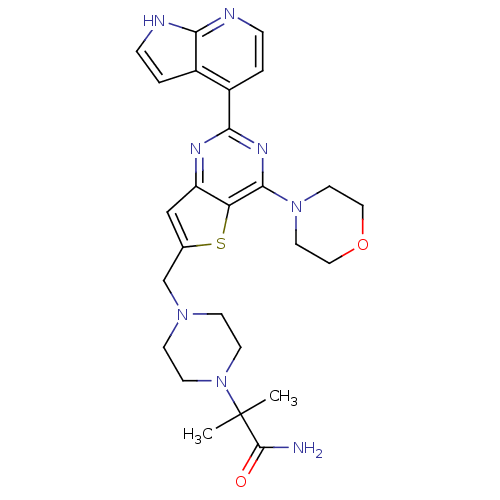
(CHEMBL2165513)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2ccnc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-9-7-32(8-10-34)16-17-15-20-21(37-17)24(33-11-13-36-14-12-33)31-23(30-20)19-4-6-29-22-18(19)3-5-28-22/h3-6,15H,7-14,16H2,1-2H3,(H2,27,35)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data