

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897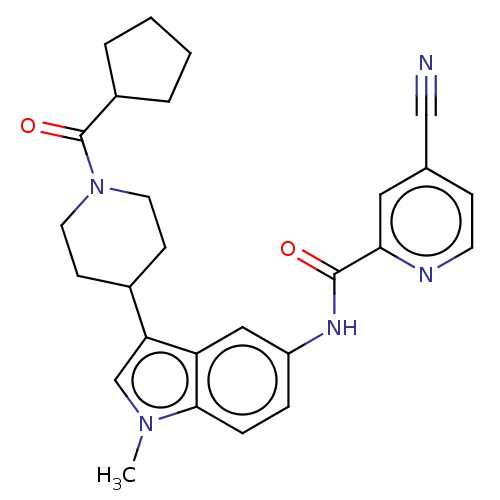 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891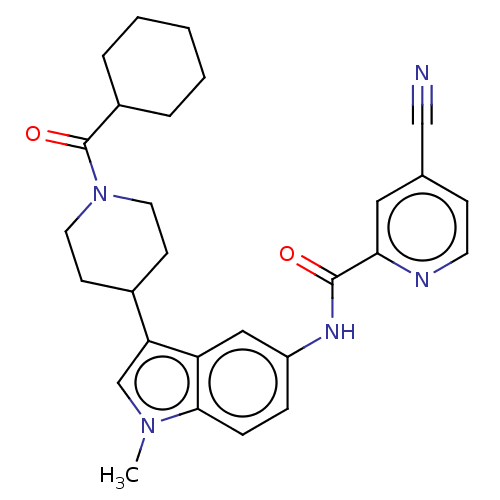 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893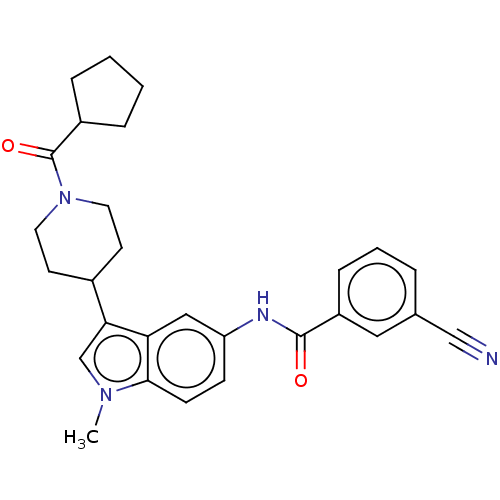 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892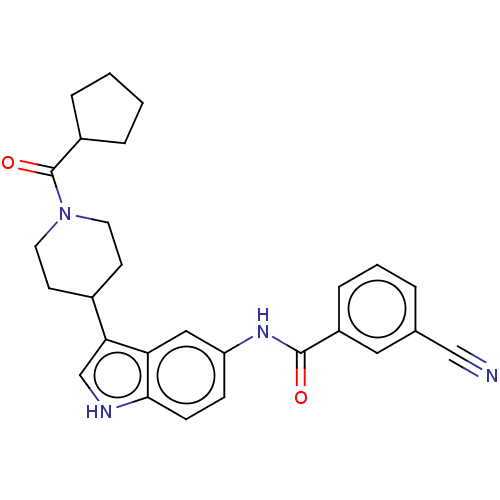 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466913 (CHEMBL4289304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM50464991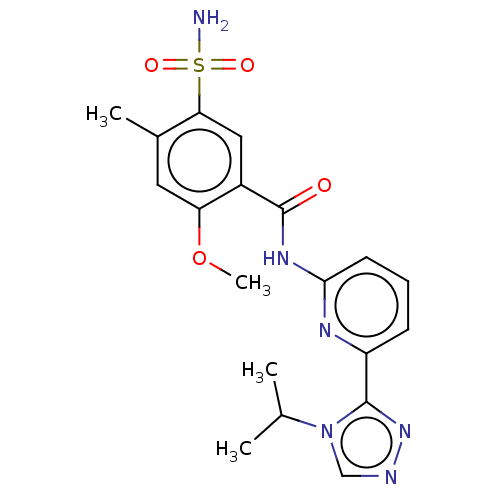 (CHEMBL4283739) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length human GST-tagged ASK1 expressed in baculovirus expression system using STK3 peptide substrate preincubated for ... | Eur J Med Chem 145: 606-621 (2018) Article DOI: 10.1016/j.ejmech.2017.12.041 BindingDB Entry DOI: 10.7270/Q25H7JXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500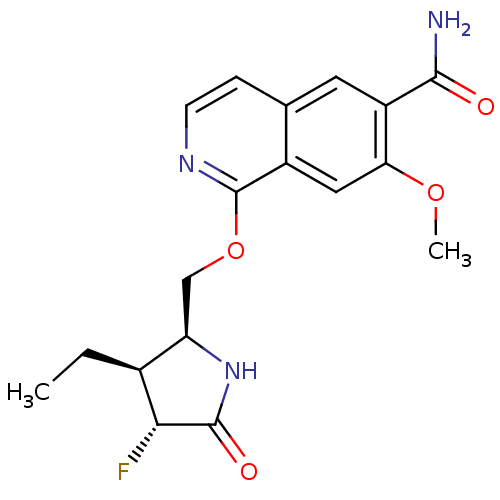 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403204 (US10329302, Example 360 | US10793579, Example 360 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM319575 (5-(((2S,3S,4S)-3-ethyl-4-fluoro-5-oxopyrrolidin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | US Patent US10174000 (2019) BindingDB Entry DOI: 10.7270/Q27S7QWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403204 (US10329302, Example 360 | US10793579, Example 360 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403214 (US10329302, Example 370 | US10793579, Example 370 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403204 (US10329302, Example 360 | US10793579, Example 360 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403214 (US10329302, Example 370 | US10793579, Example 370 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403214 (US10329302, Example 370 | US10793579, Example 370 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470468 (1-[(3R)-1-acryloylpiperidin-3-yl]-5-amino-3-[4-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377836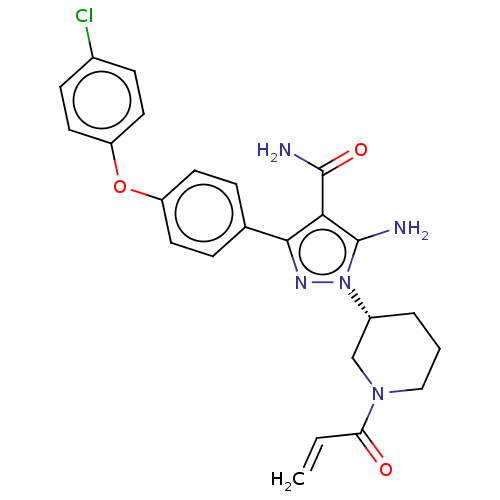 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303543 (US10138229, Example 54 | US10266513, Example 127 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303543 (US10138229, Example 54 | US10266513, Example 127 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377836 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM402999 (US10329302, Example 146 | US10793579, Example 146 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM402999 (US10329302, Example 146 | US10793579, Example 146 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403189 (US10329302, Example 345 | US10793579, Example 345 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403169 (US10329302, Example 323 | US10793579, Example 323 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403035 (US10329302, Example 183 | US10793579, Example 183 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403169 (US10329302, Example 323 | US10793579, Example 323 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403189 (US10329302, Example 345 | US10793579, Example 345 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403189 (US10329302, Example 345 | US10793579, Example 345 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403169 (US10329302, Example 323 | US10793579, Example 323 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM402999 (US10329302, Example 146 | US10793579, Example 146 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403035 (US10329302, Example 183 | US10793579, Example 183 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403035 (US10329302, Example 183 | US10793579, Example 183 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377842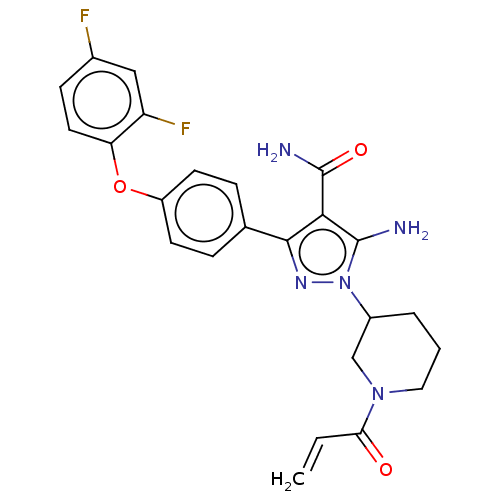 (1-[(3S)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377842 (1-[(3S)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2239 total ) | Next | Last >> |