 Found 77 hits with Last Name = 'zeller' and Initial = 'sl'
Found 77 hits with Last Name = 'zeller' and Initial = 'sl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50101331
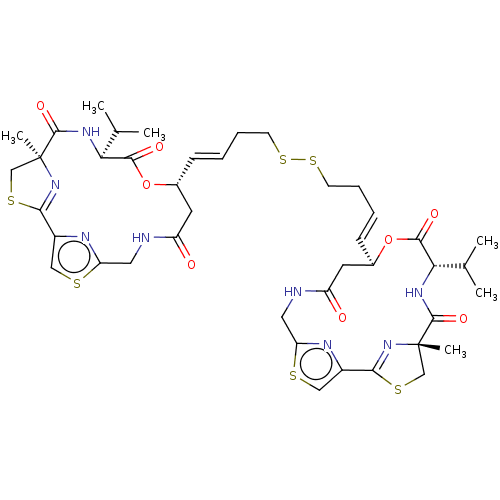
(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055512
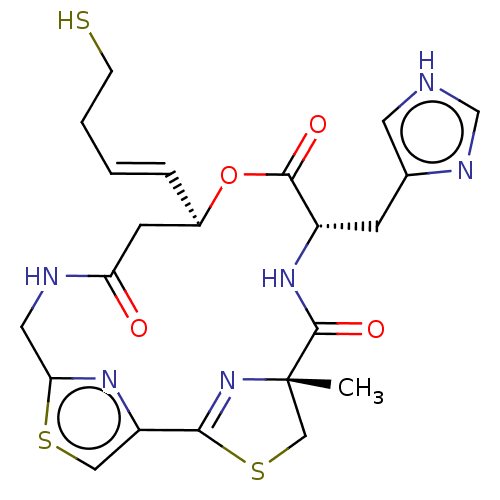
(CHEMBL3317812)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3c[nH]cn3)NC2=O)\C=C\CCS)n1 |r,c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50101330
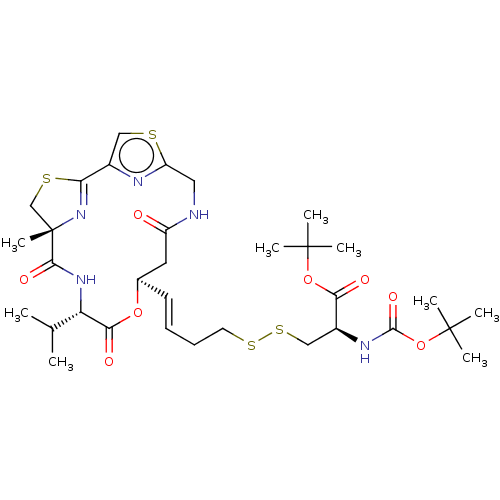
(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50101331

(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055513
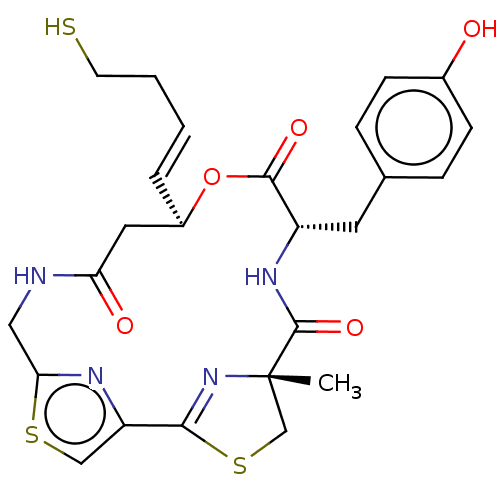
(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055514
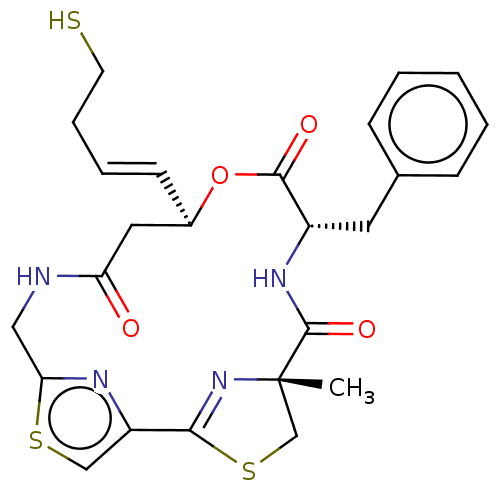
(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50101331

(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50101331

(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055513

(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50101330

(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50098414

(CHEMBL3593247)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:11| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length C-terminal His/FLAG-tagged human HDAC1 expressed in baculovirus infected Sf9 insect cells using BPS HDAC substr... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50101331

(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50098414

(CHEMBL3593247)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:11| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17+,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC10 (1 to 481 residues) expressed in baculovirus infected Sf9 insect cells using B... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50101330

(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50101330

(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055515

(CHEMBL3317818)Show SMILES CC(C)(C)OC(=O)n1cnc(C[C@@H]2NC(=O)[C@]3(C)CSC(=N3)c3csc(CNC(=O)C[C@H](OC2=O)\C=C\CCS)n3)c1 |r,c:20| Show InChI InChI=1S/C27H34N6O6S3/c1-26(2,3)39-25(37)33-12-16(29-15-33)9-18-23(35)38-17(7-5-6-8-40)10-20(34)28-11-21-30-19(13-41-21)22-32-27(4,14-42-22)24(36)31-18/h5,7,12-13,15,17-18,40H,6,8-11,14H2,1-4H3,(H,28,34)(H,31,36)/b7-5+/t17-,18+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055514

(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50098414

(CHEMBL3593247)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:11| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of full length human C-terminal His-tagged HDAC3/N-terminal GST-tagged NCOR2 (395 to 489 residues) expressed in baculovirus infected Sf9 i... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50098414

(CHEMBL3593247)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:11| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of full length C-terminal FLAG-tagged human HDAC2 expressed in baculovirus infected Sf9 insect cells using BPS HDAC substrate 3 after 30 m... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50101330

(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055516
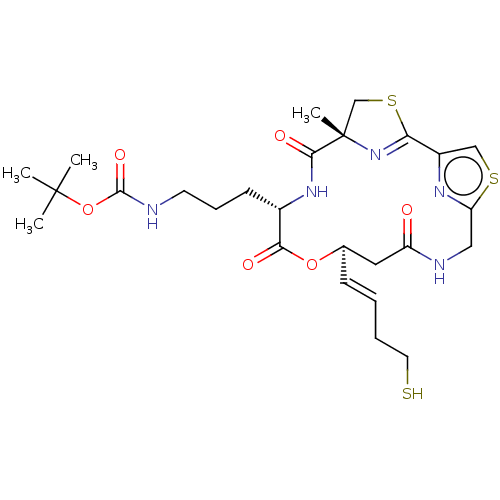
(CHEMBL3317817)Show SMILES CC(C)(C)OC(=O)NCCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:19| Show InChI InChI=1S/C26H37N5O6S3/c1-25(2,3)37-24(35)27-10-7-9-17-22(33)36-16(8-5-6-11-38)12-19(32)28-13-20-29-18(14-39-20)21-31-26(4,15-40-21)23(34)30-17/h5,8,14,16-17,38H,6-7,9-13,15H2,1-4H3,(H,27,35)(H,28,32)(H,30,34)/b8-5+/t16-,17+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055513

(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055512

(CHEMBL3317812)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3c[nH]cn3)NC2=O)\C=C\CCS)n1 |r,c:4| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055515

(CHEMBL3317818)Show SMILES CC(C)(C)OC(=O)n1cnc(C[C@@H]2NC(=O)[C@]3(C)CSC(=N3)c3csc(CNC(=O)C[C@H](OC2=O)\C=C\CCS)n3)c1 |r,c:20| Show InChI InChI=1S/C27H34N6O6S3/c1-26(2,3)39-25(37)33-12-16(29-15-33)9-18-23(35)38-17(7-5-6-8-40)10-20(34)28-11-21-30-19(13-41-21)22-32-27(4,14-42-22)24(36)31-18/h5,7,12-13,15,17-18,40H,6,8-11,14H2,1-4H3,(H,28,34)(H,31,36)/b7-5+/t17-,18+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055515

(CHEMBL3317818)Show SMILES CC(C)(C)OC(=O)n1cnc(C[C@@H]2NC(=O)[C@]3(C)CSC(=N3)c3csc(CNC(=O)C[C@H](OC2=O)\C=C\CCS)n3)c1 |r,c:20| Show InChI InChI=1S/C27H34N6O6S3/c1-26(2,3)39-25(37)33-12-16(29-15-33)9-18-23(35)38-17(7-5-6-8-40)10-20(34)28-11-21-30-19(13-41-21)22-32-27(4,14-42-22)24(36)31-18/h5,7,12-13,15,17-18,40H,6,8-11,14H2,1-4H3,(H,28,34)(H,31,36)/b7-5+/t17-,18+,27+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055516

(CHEMBL3317817)Show SMILES CC(C)(C)OC(=O)NCCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:19| Show InChI InChI=1S/C26H37N5O6S3/c1-25(2,3)37-24(35)27-10-7-9-17-22(33)36-16(8-5-6-11-38)12-19(32)28-13-20-29-18(14-39-20)21-31-26(4,15-40-21)23(34)30-17/h5,8,14,16-17,38H,6-7,9-13,15H2,1-4H3,(H,27,35)(H,28,32)(H,30,34)/b8-5+/t16-,17+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055512

(CHEMBL3317812)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3c[nH]cn3)NC2=O)\C=C\CCS)n1 |r,c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055514

(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055516

(CHEMBL3317817)Show SMILES CC(C)(C)OC(=O)NCCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:19| Show InChI InChI=1S/C26H37N5O6S3/c1-25(2,3)37-24(35)27-10-7-9-17-22(33)36-16(8-5-6-11-38)12-19(32)28-13-20-29-18(14-39-20)21-31-26(4,15-40-21)23(34)30-17/h5,8,14,16-17,38H,6-7,9-13,15H2,1-4H3,(H,27,35)(H,28,32)(H,30,34)/b8-5+/t16-,17+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055517
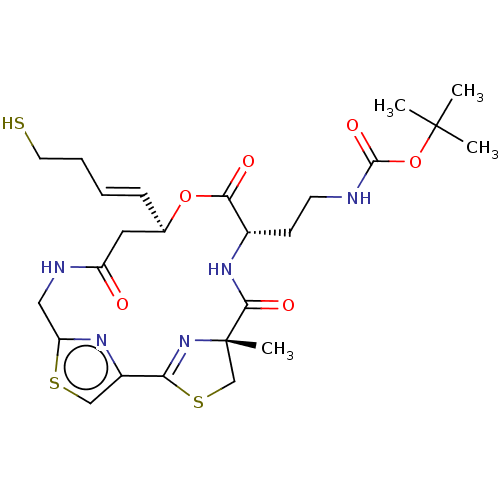
(CHEMBL3317816)Show SMILES CC(C)(C)OC(=O)NCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:18| Show InChI InChI=1S/C25H35N5O6S3/c1-24(2,3)36-23(34)26-9-8-16-21(32)35-15(7-5-6-10-37)11-18(31)27-12-19-28-17(13-38-19)20-30-25(4,14-39-20)22(33)29-16/h5,7,13,15-16,37H,6,8-12,14H2,1-4H3,(H,26,34)(H,27,31)(H,29,33)/b7-5+/t15-,16+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055517

(CHEMBL3317816)Show SMILES CC(C)(C)OC(=O)NCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:18| Show InChI InChI=1S/C25H35N5O6S3/c1-24(2,3)36-23(34)26-9-8-16-21(32)35-15(7-5-6-10-37)11-18(31)27-12-19-28-17(13-38-19)20-30-25(4,14-39-20)22(33)29-16/h5,7,13,15-16,37H,6,8-12,14H2,1-4H3,(H,26,34)(H,27,31)(H,29,33)/b7-5+/t15-,16+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055518

(CHEMBL3317815)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C21H29N5O4S3/c1-21-12-33-18(26-21)15-11-32-17(24-15)10-23-16(27)9-13(5-2-3-8-31)30-19(28)14(6-4-7-22)25-20(21)29/h2,5,11,13-14,31H,3-4,6-10,12,22H2,1H3,(H,23,27)(H,25,29)/b5-2+/t13-,14+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50242139

(CHEMBL4078721)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSCCS)n2 |r,c:11| Show InChI InChI=1S/C23H32N4O4S4/c1-14(2)19-21(29)31-15(6-4-5-8-33-9-7-32)10-17(28)24-11-18-25-16(12-34-18)20-27-23(3,13-35-20)22(30)26-19/h4,6,12,14-15,19,32H,5,7-11,13H2,1-3H3,(H,24,28)(H,26,30)/b6-4+/t15-,19+,23+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC10 (1 to 481 residues) expressed in baculovirus infected Sf9 insect cells using B... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055517

(CHEMBL3317816)Show SMILES CC(C)(C)OC(=O)NCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:18| Show InChI InChI=1S/C25H35N5O6S3/c1-24(2,3)36-23(34)26-9-8-16-21(32)35-15(7-5-6-10-37)11-18(31)27-12-19-28-17(13-38-19)20-30-25(4,14-39-20)22(33)29-16/h5,7,13,15-16,37H,6,8-12,14H2,1-4H3,(H,26,34)(H,27,31)(H,29,33)/b7-5+/t15-,16+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055510

(CHEMBL3317814)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C20H27N5O4S3/c1-20-11-32-17(25-20)14-10-31-16(23-14)9-22-15(26)8-12(4-2-3-7-30)29-18(27)13(5-6-21)24-19(20)28/h2,4,10,12-13,30H,3,5-9,11,21H2,1H3,(H,22,26)(H,24,28)/b4-2+/t12-,13+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055518

(CHEMBL3317815)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C21H29N5O4S3/c1-21-12-33-18(26-21)15-11-32-17(24-15)10-23-16(27)9-13(5-2-3-8-31)30-19(28)14(6-4-7-22)25-20(21)29/h2,5,11,13-14,31H,3-4,6-10,12,22H2,1H3,(H,23,27)(H,25,29)/b5-2+/t13-,14+,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055518

(CHEMBL3317815)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C21H29N5O4S3/c1-21-12-33-18(26-21)15-11-32-17(24-15)10-23-16(27)9-13(5-2-3-8-31)30-19(28)14(6-4-7-22)25-20(21)29/h2,5,11,13-14,31H,3-4,6-10,12,22H2,1H3,(H,23,27)(H,25,29)/b5-2+/t13-,14+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data