

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50379185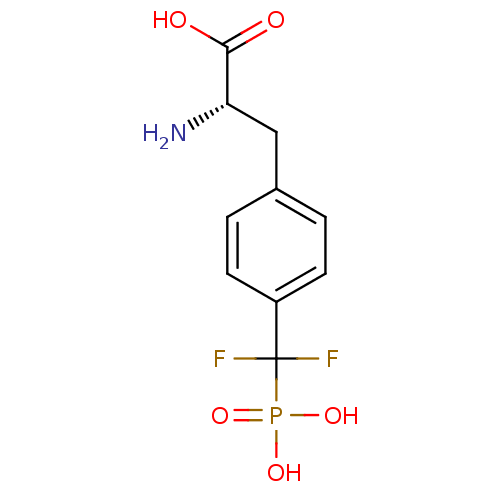 (CHEMBL1232859) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of PTP1B expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate after 2 to 3 mins by spectrophotometric... | Bioorg Med Chem 20: 1940-6 (2012) Article DOI: 10.1016/j.bmc.2011.11.004 BindingDB Entry DOI: 10.7270/Q23J3DZQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50112356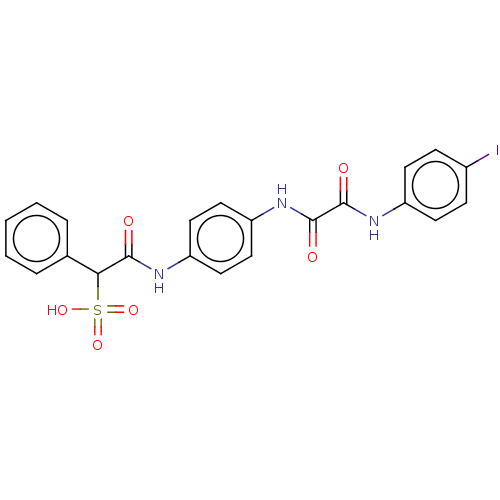 (CHEMBL3609373) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50420258 (CEFSULODIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50379182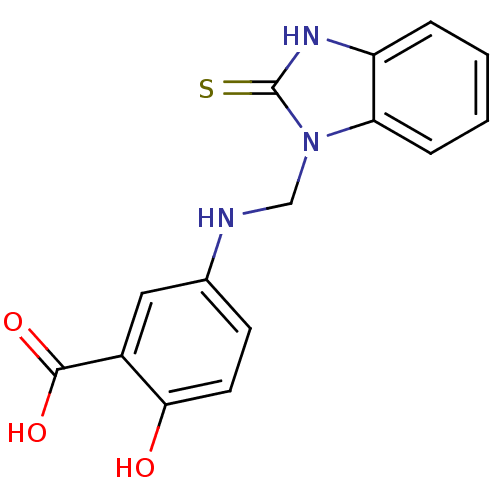 (CHEMBL289069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of PTP1B expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate after 2 to 3 mins by spectrophotometric... | Bioorg Med Chem 20: 1940-6 (2012) Article DOI: 10.1016/j.bmc.2011.11.004 BindingDB Entry DOI: 10.7270/Q23J3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine-phosphatase (Yersinia pestis) | BDBM26193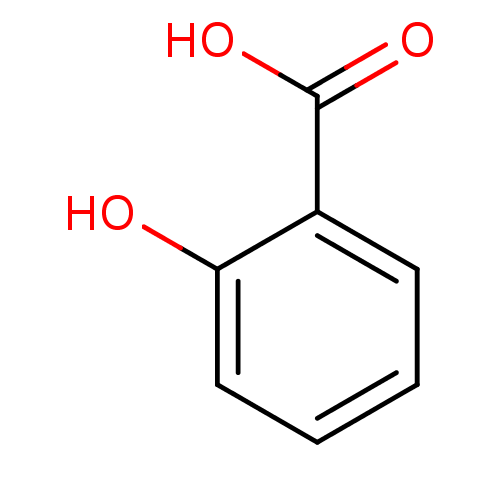 (2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of Yersinia pseudotuberculosis YopH | Bioorg Med Chem 20: 1940-6 (2012) Article DOI: 10.1016/j.bmc.2011.11.004 BindingDB Entry DOI: 10.7270/Q23J3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM26193 (2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.94E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of PTP1B expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate after 2 to 3 mins by spectrophotometric... | Bioorg Med Chem 20: 1940-6 (2012) Article DOI: 10.1016/j.bmc.2011.11.004 BindingDB Entry DOI: 10.7270/Q23J3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485904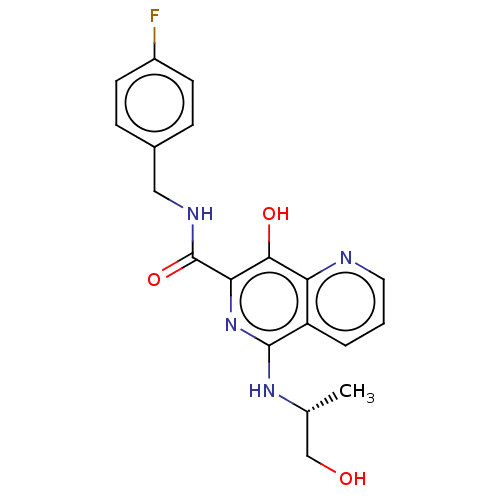 (CHEMBL2180591) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485906 (CHEMBL2180587) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054344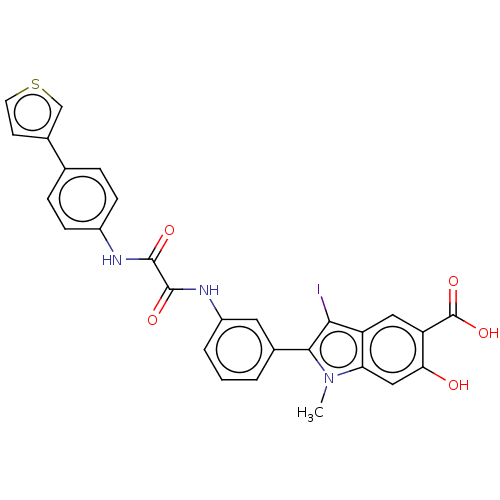 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054257 (CHEMBL3319376 | US9522881, 11a-21 L97L08 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485914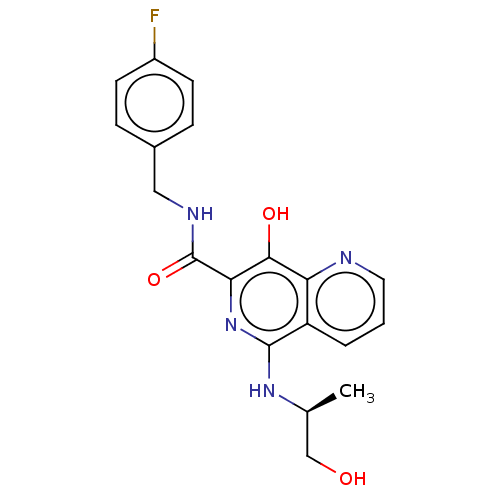 (CHEMBL2180590) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485905 (CHEMBL2180589) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50021581 (CHEMBL414850 | L-870810) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054258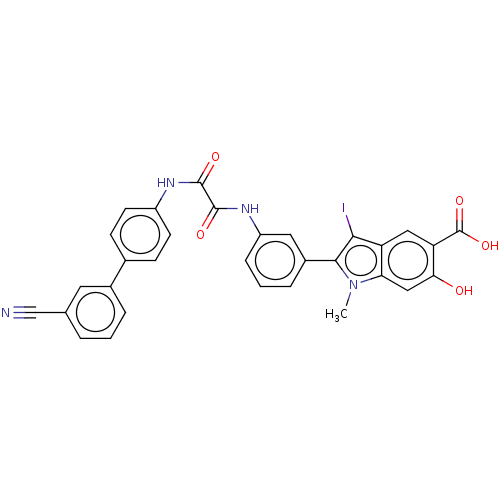 (CHEMBL3319377 | US9522881, 11a-22 L97L07 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054259 (CHEMBL3319378 | US9522881, 11a-23 L97L03) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054260 (CHEMBL3319379 | US9522881, 11a-24 L97L05 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479066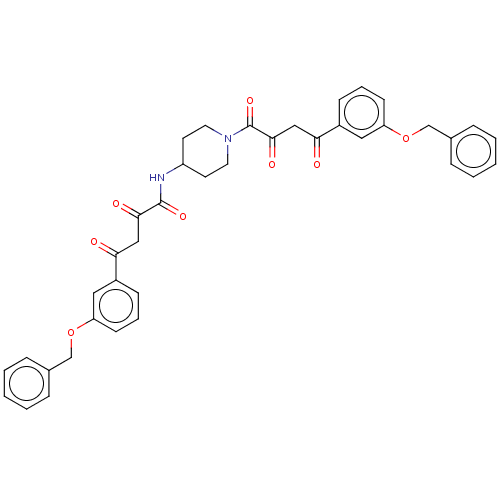 (CHEMBL454190) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479075 (CHEMBL492770) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479081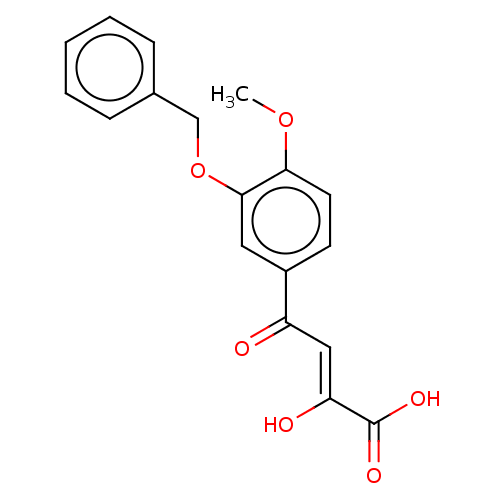 (CHEMBL467149) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054261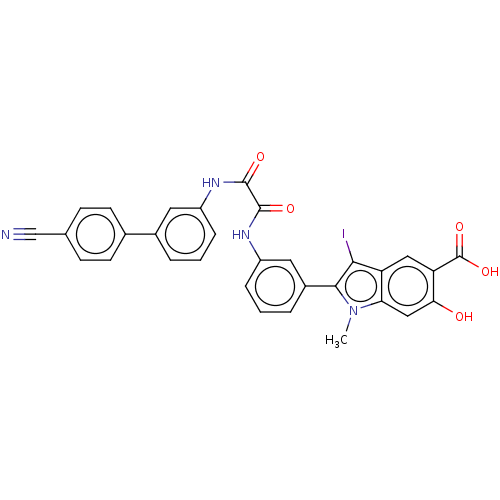 (CHEMBL3319380 | US9522881, 11a-25 L97L06 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485907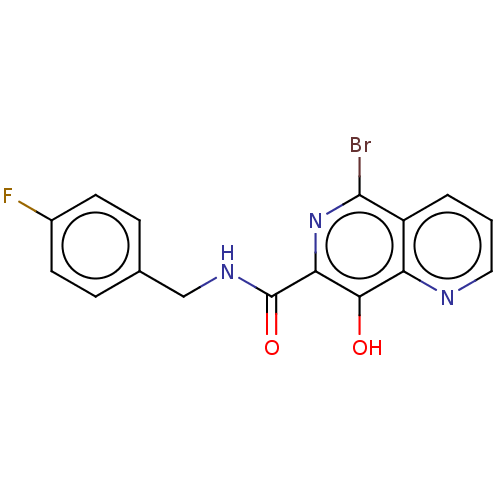 (CHEMBL2180599) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479058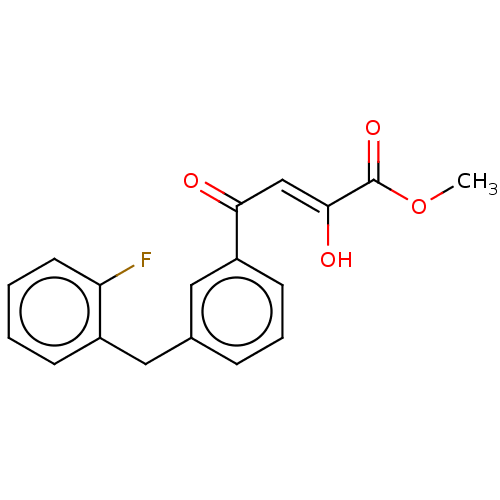 (CHEMBL467380) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054406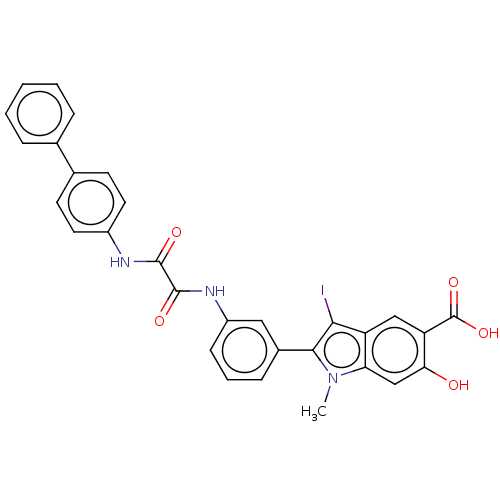 (CHEMBL3319357 | US9522881, 11a-2 (L97N08) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054262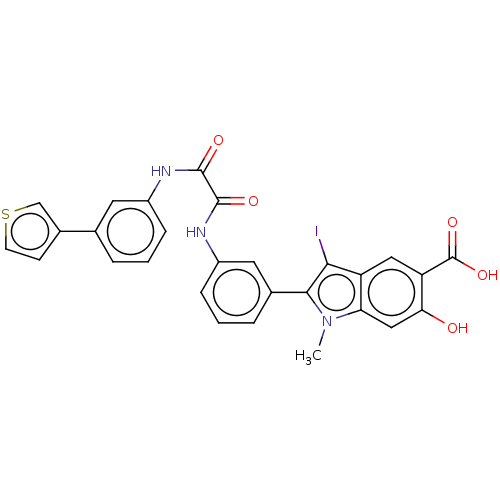 (CHEMBL3319381 | US9522881, 11a-26 L97L02 | US98445...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054405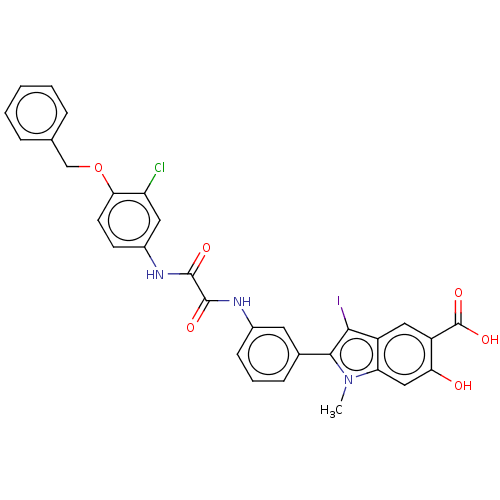 (CHEMBL3319358 | US9522881, 11a-3 (L97M50) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485913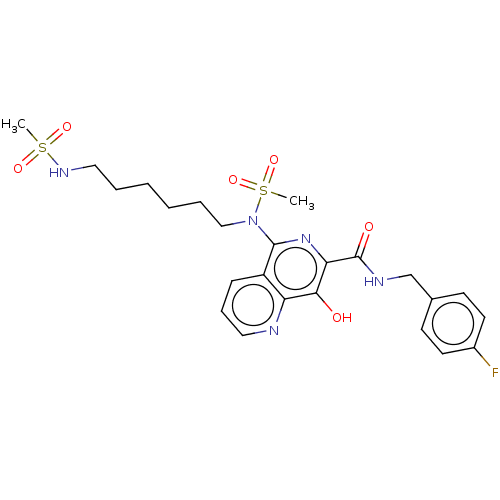 (CHEMBL2180586) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485917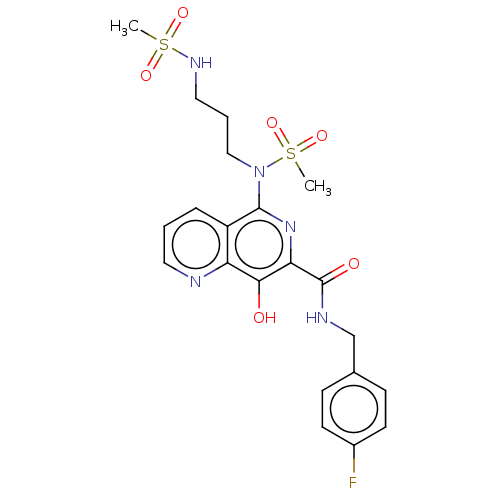 (CHEMBL2180585) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054404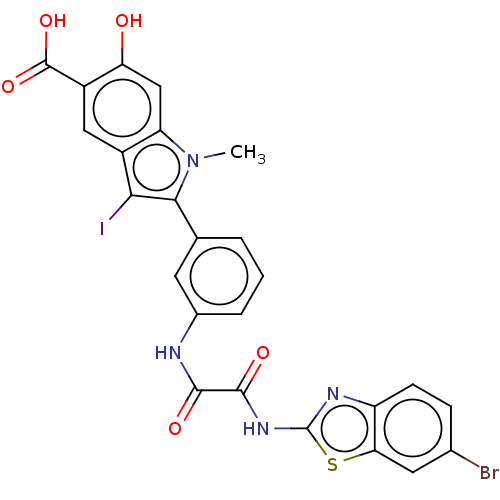 (CHEMBL3319359 | US9522881, 11a-4 (L97M61) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054403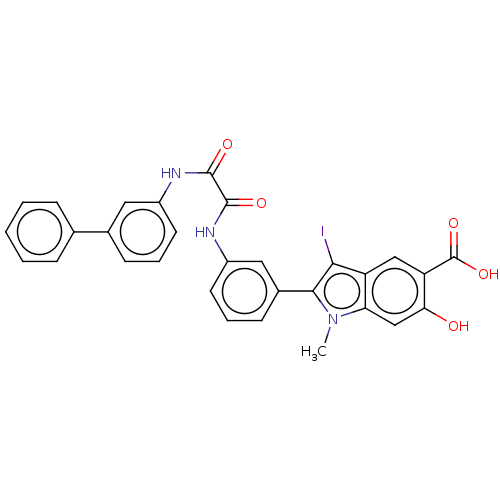 (CHEMBL3319360 | US9522881, 11a-5 (L97M48) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054402 (CHEMBL3319361 | US9522881, 11a-6 (L97M52) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485911 (CHEMBL2180588) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 7 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HePTP (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 22 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of LYP (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054401 (CHEMBL3319362 | US9522881, 11a-7 (L97M93) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 3 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPH1 (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054400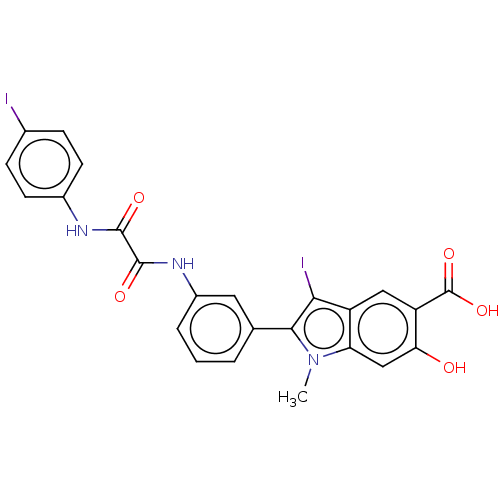 (CHEMBL3319363 | US9522881, 11a-8 (L97M24) | US9844...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054399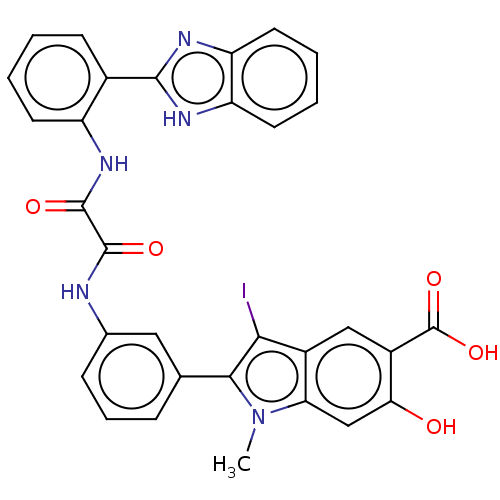 (CHEMBL3319364 | US9522881, 11a-9 (L97M77)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM50341986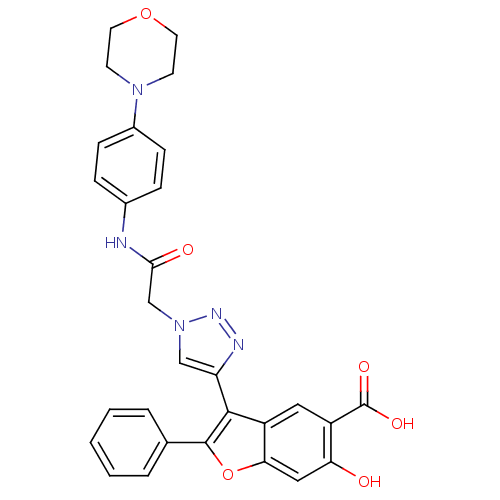 (6-hydroxy-3-(1-(2-(4-morpholinophenylamino)-2-oxoe...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis PTPB | Bioorg Med Chem 20: 1940-6 (2012) Article DOI: 10.1016/j.bmc.2011.11.004 BindingDB Entry DOI: 10.7270/Q23J3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA polymerase II subunit A C-terminal domain phosphatase SSU72 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Ssu72 (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054350 (CHEMBL3319365 | US9522881, 11a-10 (L97N15) | US984...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50112358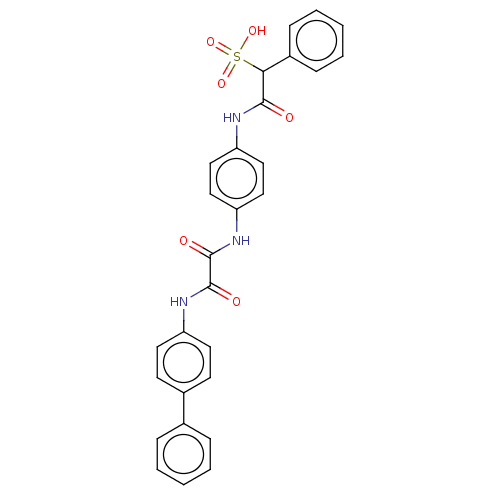 (CHEMBL3609374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP1 (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054349 (CHEMBL3319366 | US9522881, 11a-11 (L97M21) | US984...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054348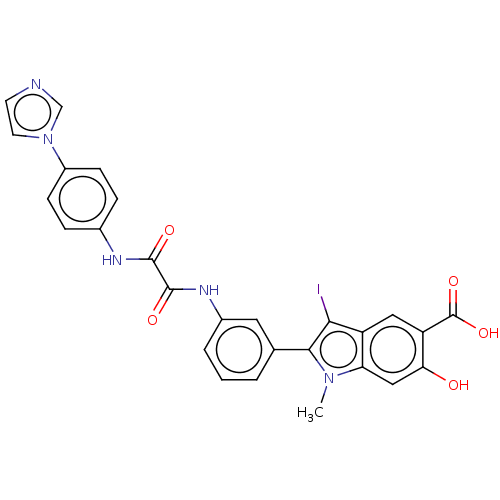 (CHEMBL3319367 | US9522881, 11a-12 (L97M63) | US984...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50112356 (CHEMBL3609373) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054347 (CHEMBL3319368 | US9522881, 11a-13 (L97M30) | US984...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054346 (CHEMBL3319369 | US9522881, 11a-14 (L97M73) | US984...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479065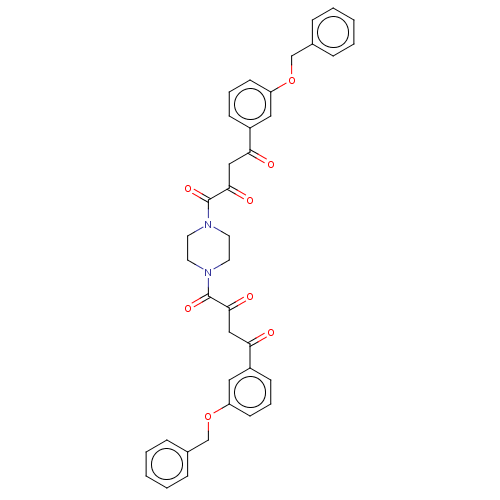 (CHEMBL443890) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50054345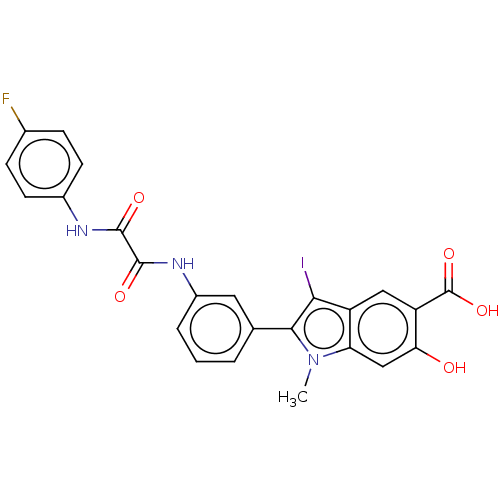 (CHEMBL3319370 | US9522881, 11a-15 (L97N95) | US984...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 344 total ) | Next | Last >> |