 Found 165 hits with Last Name = 'zheng' and Initial = 'd'
Found 165 hits with Last Name = 'zheng' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633586

(N—((S)-1-cyano-2-(3-fluoro-4′-(pentafluoro-1...)Show SMILES CO[C@@H]1CNC[C@H](OC1)C(=O)N[C@@H](Cc1ccc(cc1F)-c1ccc(cc1)S(F)(F)(F)(F)F)C#N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633583

((S)—N—((S)-1-cyano-2-(2-fluoro-4-(3-(methyl-d3)-2-...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)c(F)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144428

(CHEMBL66159 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NN Show InChI InChI=1S/C26H32FN5O3/c1-26(2,27)13-12-18(24(34)32-28)15-23(33)21(14-17-8-4-3-5-9-17)31-25(35)22-16-29-19-10-6-7-11-20(19)30-22/h3-11,16,18,21,23,33H,12-15,28H2,1-2H3,(H,31,35)(H,32,34)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633575
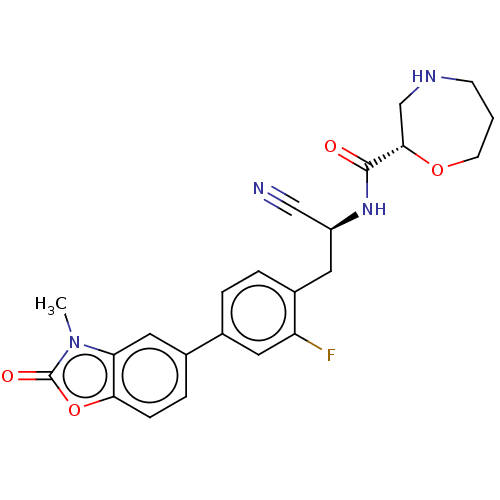
((S)—N—((S)-1-cyano-2-(2-fluoro-4-(3-methyl-2-oxo-2...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)c(F)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144400

(CHEMBL304358 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H32N4O3/c1-17(2)12-13-19(25(27)32)15-24(31)22(14-18-8-4-3-5-9-18)30-26(33)23-16-28-20-10-6-7-11-21(20)29-23/h3-11,16-17,19,22,24,31H,12-15H2,1-2H3,(H2,27,32)(H,30,33)/t19-,22+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633585
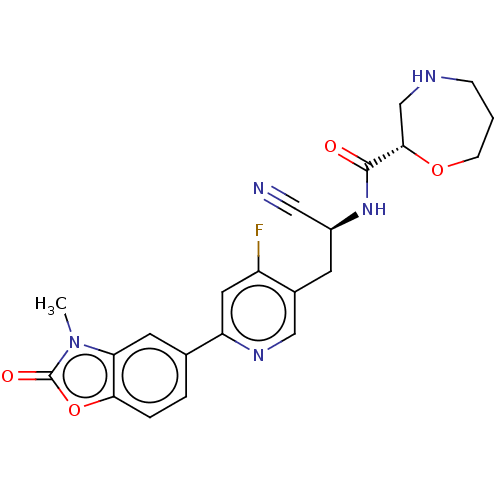
(US11807635, Compound 35)Show SMILES Cn1c2cc(ccc2oc1=O)-c1cc(F)c(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cn1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633584
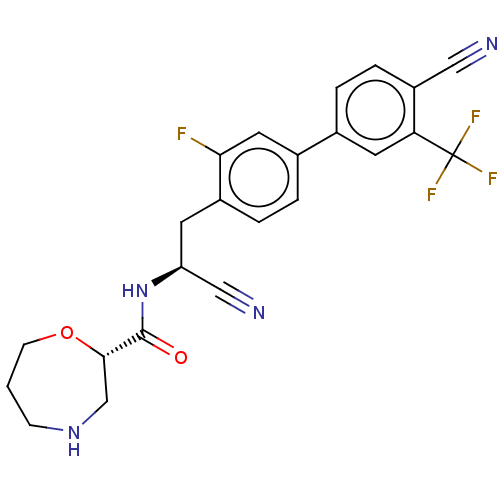
((S)—N—((S)-1-cyano-2-(4′-cyano-3-fluoro-3�...)Show SMILES Fc1cc(ccc1C[C@H](NC(=O)[C@@H]1CNCCCO1)C#N)-c1ccc(C#N)c(c1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144420
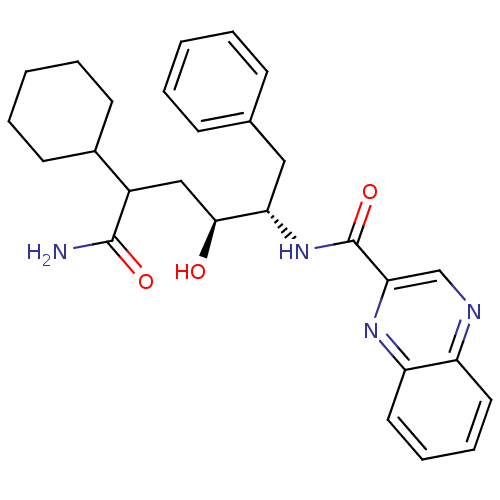
(CHEMBL68145 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCCCC1 Show InChI InChI=1S/C27H32N4O3/c28-26(33)20(19-11-5-2-6-12-19)16-25(32)23(15-18-9-3-1-4-10-18)31-27(34)24-17-29-21-13-7-8-14-22(21)30-24/h1,3-4,7-10,13-14,17,19-20,23,25,32H,2,5-6,11-12,15-16H2,(H2,28,33)(H,31,34)/t20?,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633576
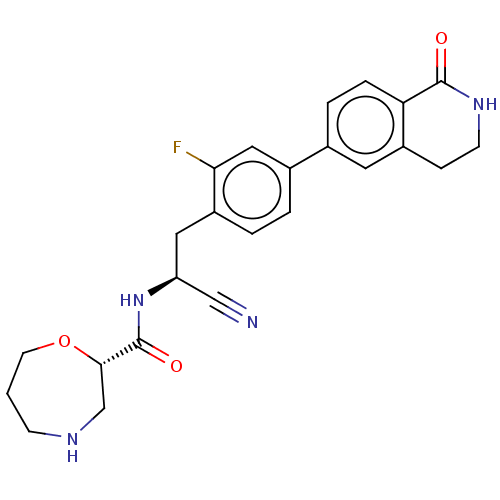
((S)—N—((S)-1-cyano-2-(2-fluoro-4-(1-oxo-1,2,3,4-te...)Show SMILES Fc1cc(ccc1C[C@H](NC(=O)[C@@H]1CNCCCO1)C#N)-c1ccc2C(=O)NCCc2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144428

(CHEMBL66159 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NN Show InChI InChI=1S/C26H32FN5O3/c1-26(2,27)13-12-18(24(34)32-28)15-23(33)21(14-17-8-4-3-5-9-17)31-25(35)22-16-29-19-10-6-7-11-20(19)30-22/h3-11,16,18,21,23,33H,12-15,28H2,1-2H3,(H,31,35)(H,32,34)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144431

(CHEMBL303673 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES ONC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O4/c28-27(29)12-10-18(11-13-27)19(25(35)33-37)15-24(34)22(14-17-6-2-1-3-7-17)32-26(36)23-16-30-20-8-4-5-9-21(20)31-23/h1-9,16,18-19,22,24,34,37H,10-15H2,(H,32,36)(H,33,35)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633582
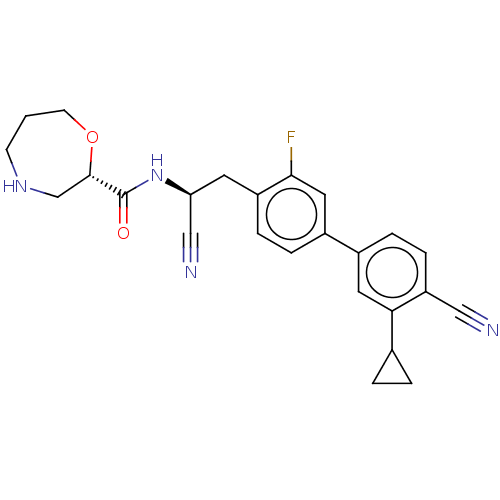
((S)—N—((S)-1-cyano-2-(4′-cyano-3′-cycl...)Show SMILES Fc1cc(ccc1C[C@H](NC(=O)[C@@H]1CNCCCO1)C#N)-c1ccc(C#N)c(c1)C1CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144431

(CHEMBL303673 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES ONC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O4/c28-27(29)12-10-18(11-13-27)19(25(35)33-37)15-24(34)22(14-17-6-2-1-3-7-17)32-26(36)23-16-30-20-8-4-5-9-21(20)31-23/h1-9,16,18-19,22,24,34,37H,10-15H2,(H,32,36)(H,33,35)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144396

(CHEMBL69375 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES NC(=O)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCCCC1 Show InChI InChI=1S/C27H32N4O3/c28-26(33)20(19-11-5-2-6-12-19)16-25(32)23(15-18-9-3-1-4-10-18)31-27(34)24-17-29-21-13-7-8-14-22(21)30-24/h1,3-4,7-10,13-14,17,19-20,23,25,32H,2,5-6,11-12,15-16H2,(H2,28,33)(H,31,34)/t20-,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144420

(CHEMBL68145 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCCCC1 Show InChI InChI=1S/C27H32N4O3/c28-26(33)20(19-11-5-2-6-12-19)16-25(32)23(15-18-9-3-1-4-10-18)31-27(34)24-17-29-21-13-7-8-14-22(21)30-24/h1,3-4,7-10,13-14,17,19-20,23,25,32H,2,5-6,11-12,15-16H2,(H2,28,33)(H,31,34)/t20?,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144417
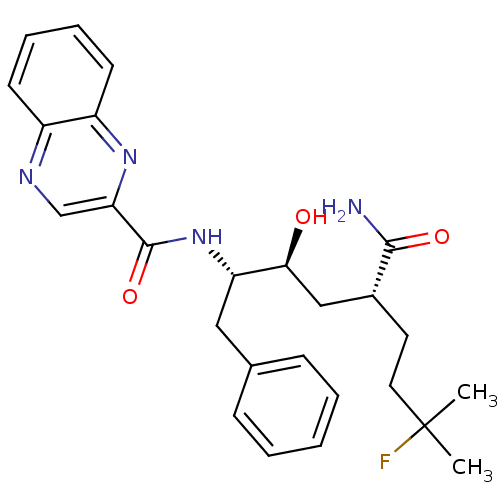
(CHEMBL68366 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CC(C)(F)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O3/c1-26(2,27)13-12-18(24(28)33)15-23(32)21(14-17-8-4-3-5-9-17)31-25(34)22-16-29-19-10-6-7-11-20(19)30-22/h3-11,16,18,21,23,32H,12-15H2,1-2H3,(H2,28,33)(H,31,34)/t18-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555231

(CHEMBL4762103)Show SMILES C[C@@H](c1cc(OCC(F)F)ccn1)n1cnc2c(N)nc(Cl)nc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633580
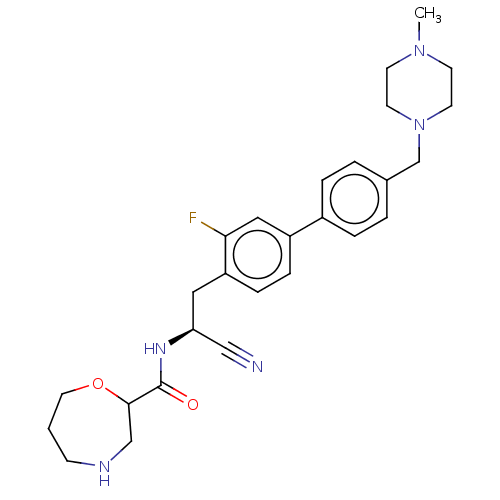
((S)—N—((S)-1-cyano-2-(3-fluoro-4′-((4-methyl...)Show SMILES CN1CCN(Cc2ccc(cc2)-c2ccc(C[C@H](NC(=O)C3CNCCCO3)C#N)c(F)c2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144417

(CHEMBL68366 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CC(C)(F)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O3/c1-26(2,27)13-12-18(24(28)33)15-23(32)21(14-17-8-4-3-5-9-17)31-25(34)22-16-29-19-10-6-7-11-20(19)30-22/h3-11,16,18,21,23,32H,12-15H2,1-2H3,(H2,28,33)(H,31,34)/t18-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144412
(CHEMBL69659 | Quinoxaline-2-carboxylic acid [(1S,2...)Show SMILES CC1CC(CC(C)O1)[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C28H34N4O4/c1-17-12-20(13-18(2)36-17)21(27(29)34)15-26(33)24(14-19-8-4-3-5-9-19)32-28(35)25-16-30-22-10-6-7-11-23(22)31-25/h3-11,16-18,20-21,24,26,33H,12-15H2,1-2H3,(H2,29,34)(H,32,35)/t17?,18?,20?,21-,24-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
Major capsid protein L1
(Human papillomavirus type 16) | BDBM50089219
(CHEMBL3577826)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H124N20O21/c1-42(2)33-53(97-76(117)60-25-18-32-99(60)77(118)59(38-47-21-13-10-14-22-47)98-70(111)51(26-28-61(82)100)90-75(116)58(40-65(105)106)96-72(113)54(34-43(3)4)92-66(107)48(81)39-64(103)104)67(108)87-41-63(102)88-49(24-17-31-86-79(84)85)68(109)89-50(23-15-16-30-80)69(110)95-57(37-46-19-11-9-12-20-46)74(115)94-56(36-45(7)8)73(114)93-55(35-44(5)6)71(112)91-52(78(119)120)27-29-62(83)101/h9-14,19-22,42-45,48-60H,15-18,23-41,80-81H2,1-8H3,(H2,82,100)(H2,83,101)(H,87,108)(H,88,102)(H,89,109)(H,90,116)(H,91,112)(H,92,107)(H,93,114)(H,94,115)(H,95,110)(H,96,113)(H,97,117)(H,98,111)(H,103,104)(H,105,106)(H,119,120)(H4,84,85,86)/t48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of pentamer formation of GST-tagged HPV16 major capsid protein L1 assessed as increased L1-m peak formation incubated with compound after ... |
ACS Med Chem Lett 6: 381-5 (2015)
Article DOI: 10.1021/ml500392y
BindingDB Entry DOI: 10.7270/Q2TX3H4H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144421

(CHEMBL302533 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O3/c28-27(29)12-10-18(11-13-27)19(25(30)35)15-24(34)22(14-17-6-2-1-3-7-17)33-26(36)23-16-31-20-8-4-5-9-21(20)32-23/h1-9,16,18-19,22,24,34H,10-15H2,(H2,30,35)(H,33,36)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633578
((S)—N—((S)-1-cyano-2-(4-(5-cyano-4-methylthiazol-2...)Show SMILES Cc1nc(sc1C#N)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)c(F)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144415
(CHEMBL304845 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CNC(=O)[C@H](CCC(C)C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C27H34N4O3/c1-18(2)13-14-20(26(33)28-3)16-25(32)23(15-19-9-5-4-6-10-19)31-27(34)24-17-29-21-11-7-8-12-22(21)30-24/h4-12,17-18,20,23,25,32H,13-16H2,1-3H3,(H,28,33)(H,31,34)/t20-,23+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555230
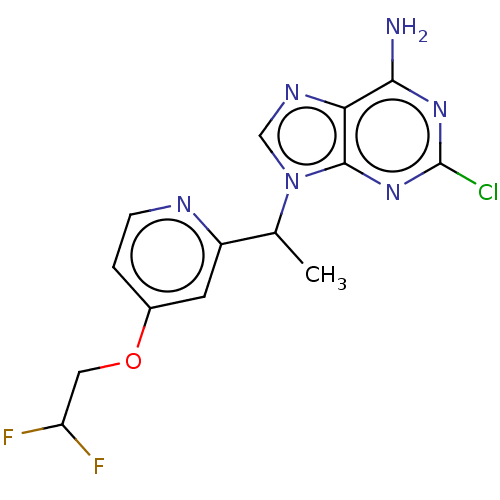
(CHEMBL4753446) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144393

(CHEMBL69274 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES NC(=O)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCCC1 Show InChI InChI=1S/C26H30N4O3/c27-25(32)19(18-10-4-5-11-18)15-24(31)22(14-17-8-2-1-3-9-17)30-26(33)23-16-28-20-12-6-7-13-21(20)29-23/h1-3,6-9,12-13,16,18-19,22,24,31H,4-5,10-11,14-15H2,(H2,27,32)(H,30,33)/t19-,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144419

(CHEMBL305423 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NO Show InChI InChI=1S/C26H31FN4O4/c1-26(2,27)13-12-18(24(33)31-35)15-23(32)21(14-17-8-4-3-5-9-17)30-25(34)22-16-28-19-10-6-7-11-20(19)29-22/h3-11,16,18,21,23,32,35H,12-15H2,1-2H3,(H,30,34)(H,31,33)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144400

(CHEMBL304358 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H32N4O3/c1-17(2)12-13-19(25(27)32)15-24(31)22(14-18-8-4-3-5-9-18)30-26(33)23-16-28-20-10-6-7-11-21(20)29-23/h3-11,16-17,19,22,24,31H,12-15H2,1-2H3,(H2,27,32)(H,30,33)/t19-,22+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144400

(CHEMBL304358 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H32N4O3/c1-17(2)12-13-19(25(27)32)15-24(31)22(14-18-8-4-3-5-9-18)30-26(33)23-16-28-20-10-6-7-11-21(20)29-23/h3-11,16-17,19,22,24,31H,12-15H2,1-2H3,(H2,27,32)(H,30,33)/t19-,22+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144419

(CHEMBL305423 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NO Show InChI InChI=1S/C26H31FN4O4/c1-26(2,27)13-12-18(24(33)31-35)15-23(32)21(14-17-8-4-3-5-9-17)30-25(34)22-16-28-19-10-6-7-11-20(19)29-22/h3-11,16,18,21,23,32,35H,12-15H2,1-2H3,(H,30,34)(H,31,33)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633581

((S)—N—((S)-1-cyano-2-(2-fluoro-4-(4-methylthiazol-...)Show SMILES Cc1csc(n1)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)c(F)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50144378
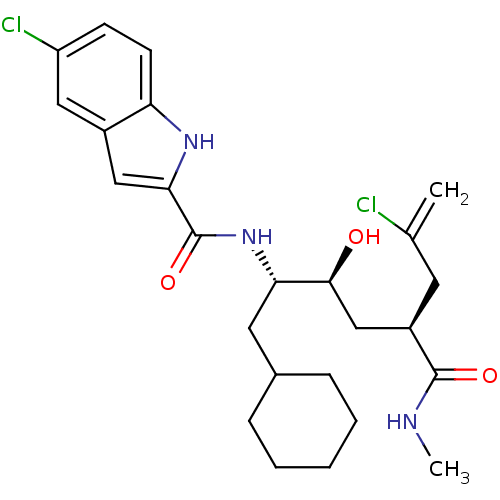
(5-Chloro-1H-indole-2-carboxylic acid ((1S,2S,4S)-6...)Show SMILES CNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1cc2cc(Cl)ccc2[nH]1)CC(Cl)=C Show InChI InChI=1S/C25H33Cl2N3O3/c1-15(26)10-18(24(32)28-2)14-23(31)21(11-16-6-4-3-5-7-16)30-25(33)22-13-17-12-19(27)8-9-20(17)29-22/h8-9,12-13,16,18,21,23,29,31H,1,3-7,10-11,14H2,2H3,(H,28,32)(H,30,33)/t18-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against renin was determined |
Bioorg Med Chem Lett 14: 2163-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.020
BindingDB Entry DOI: 10.7270/Q2CV4H5M |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555224
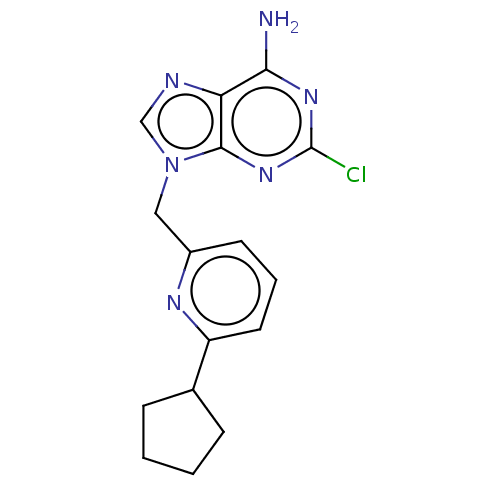
(CHEMBL4790482) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144422

(CHEMBL308473 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES CC(C)(O)CCC(C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
Major capsid protein L1
(Human papillomavirus type 16) | BDBM50089220
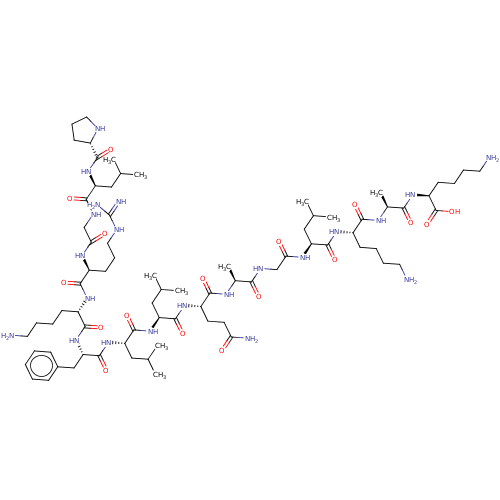
(CHEMBL3577825)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C77H134N22O17/c1-43(2)36-56(96-67(106)50-27-20-34-84-50)66(105)87-42-62(101)90-51(28-21-35-85-77(82)83)70(109)92-53(25-15-18-32-79)71(110)99-60(40-49-22-12-11-13-23-49)75(114)98-59(39-46(7)8)74(113)97-58(38-45(5)6)73(112)94-54(29-30-61(81)100)69(108)88-47(9)64(103)86-41-63(102)91-57(37-44(3)4)72(111)93-52(24-14-17-31-78)68(107)89-48(10)65(104)95-55(76(115)116)26-16-19-33-80/h11-13,22-23,43-48,50-60,84H,14-21,24-42,78-80H2,1-10H3,(H2,81,100)(H,86,103)(H,87,105)(H,88,108)(H,89,107)(H,90,101)(H,91,102)(H,92,109)(H,93,111)(H,94,112)(H,95,104)(H,96,106)(H,97,113)(H,98,114)(H,99,110)(H,115,116)(H4,82,83,85)/t47-,48-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of pentamer formation of GST-tagged HPV16 major capsid protein L1 assessed as increased L1-m peak formation incubated with compound after ... |
ACS Med Chem Lett 6: 381-5 (2015)
Article DOI: 10.1021/ml500392y
BindingDB Entry DOI: 10.7270/Q2TX3H4H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144404
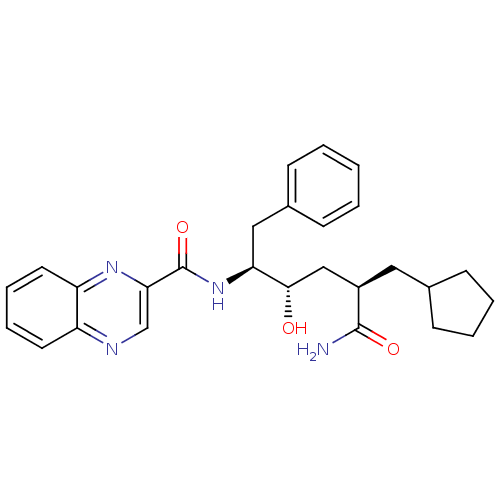
(CHEMBL306685 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES NC(=O)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)CC1CCCC1 Show InChI InChI=1S/C27H32N4O3/c28-26(33)20(14-18-10-4-5-11-18)16-25(32)23(15-19-8-2-1-3-9-19)31-27(34)24-17-29-21-12-6-7-13-22(21)30-24/h1-3,6-9,12-13,17-18,20,23,25,32H,4-5,10-11,14-16H2,(H2,28,33)(H,31,34)/t20-,23+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144425
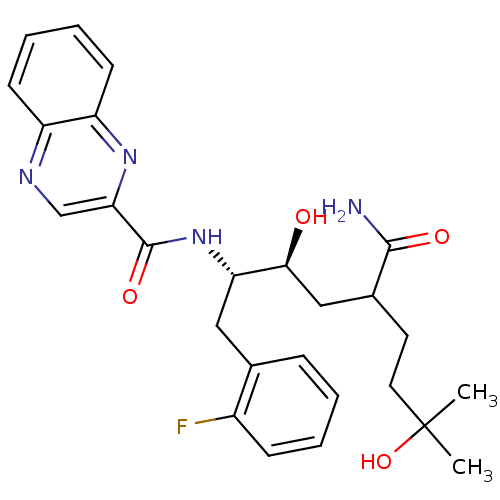
(CHEMBL304053 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES CC(C)(O)CCC(C[C@H](O)[C@H](Cc1ccccc1F)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)12-11-17(24(28)33)14-23(32)21(13-16-7-3-4-8-18(16)27)31-25(34)22-15-29-19-9-5-6-10-20(19)30-22/h3-10,15,17,21,23,32,35H,11-14H2,1-2H3,(H2,28,33)(H,31,34)/t17?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144391

(CHEMBL68581 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CNC(=O)[C@H](CC(C)C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C26H32N4O3/c1-17(2)13-19(25(32)27-3)15-24(31)22(14-18-9-5-4-6-10-18)30-26(33)23-16-28-20-11-7-8-12-21(20)29-23/h4-12,16-17,19,22,24,31H,13-15H2,1-3H3,(H,27,32)(H,30,33)/t19-,22+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144422

(CHEMBL308473 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES CC(C)(O)CCC(C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144423

(CHEMBL302320 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1(O)CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O4/c28-27(29)12-10-26(37,11-13-27)18(24(30)35)15-23(34)21(14-17-6-2-1-3-7-17)33-25(36)22-16-31-19-8-4-5-9-20(19)32-22/h1-9,16,18,21,23,34,37H,10-15H2,(H2,30,35)(H,33,36)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM633579
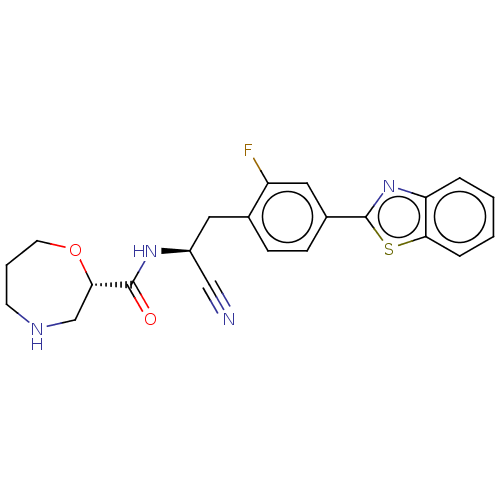
((S)—N—((S)-2-(4-(benzo[d]thiazol-2-yl)-2-fluorophe...)Show SMILES Fc1cc(ccc1C[C@H](NC(=O)[C@@H]1CNCCCO1)C#N)-c1nc2ccccc2s1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144416
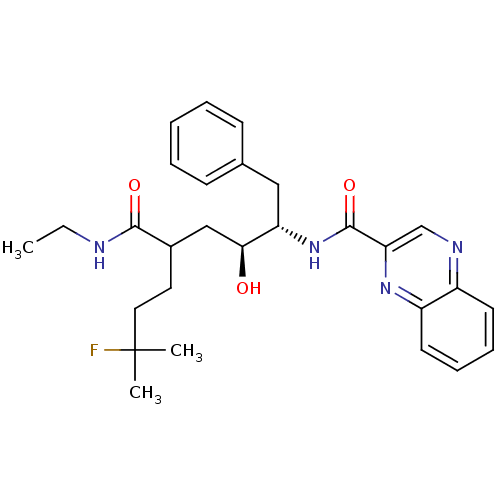
(CHEMBL69123 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CCNC(=O)C(CCC(C)(C)F)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C28H35FN4O3/c1-4-30-26(35)20(14-15-28(2,3)29)17-25(34)23(16-19-10-6-5-7-11-19)33-27(36)24-18-31-21-12-8-9-13-22(21)32-24/h5-13,18,20,23,25,34H,4,14-17H2,1-3H3,(H,30,35)(H,33,36)/t20?,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50461353
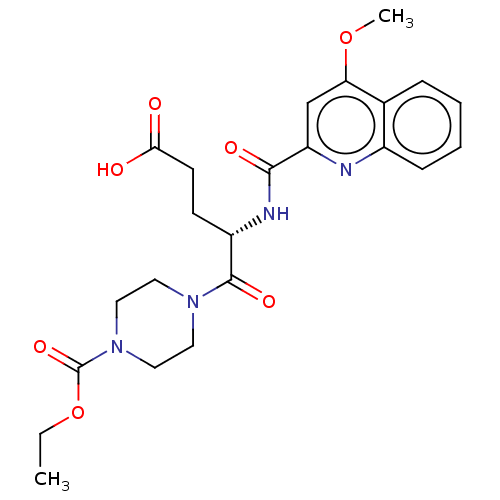
(CHEMBL4228420)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(O)=O)NC(=O)c1cc(OC)c2ccccc2n1 |r| Show InChI InChI=1S/C23H28N4O7/c1-3-34-23(32)27-12-10-26(11-13-27)22(31)17(8-9-20(28)29)25-21(30)18-14-19(33-2)15-6-4-5-7-16(15)24-18/h4-7,14,17H,3,8-13H2,1-2H3,(H,25,30)(H,28,29)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdullah International Medical Research Center/King Saud bin Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]2MeSADP from P2Y12 receptor in human platelets after 60 mins by TopCount scintillation counting method |
Bioorg Med Chem Lett 28: 1459-1463 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.090
BindingDB Entry DOI: 10.7270/Q2TF010Z |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144424
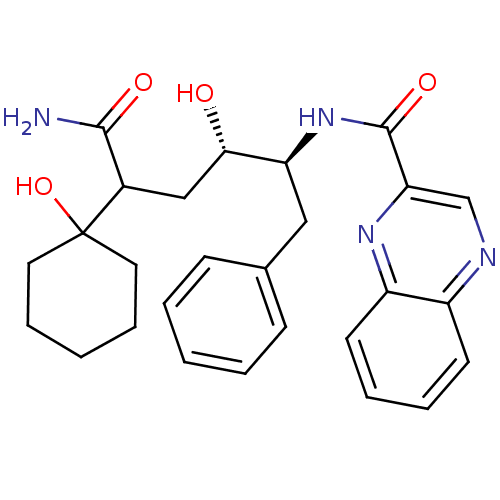
(CHEMBL306457 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1(O)CCCCC1 Show InChI InChI=1S/C27H32N4O4/c28-25(33)19(27(35)13-7-2-8-14-27)16-24(32)22(15-18-9-3-1-4-10-18)31-26(34)23-17-29-20-11-5-6-12-21(20)30-23/h1,3-6,9-12,17,19,22,24,32,35H,2,7-8,13-16H2,(H2,28,33)(H,31,34)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144416

(CHEMBL69123 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CCNC(=O)C(CCC(C)(C)F)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C28H35FN4O3/c1-4-30-26(35)20(14-15-28(2,3)29)17-25(34)23(16-19-10-6-5-7-11-19)33-27(36)24-18-31-21-12-8-9-13-22(21)32-24/h5-13,18,20,23,25,34H,4,14-17H2,1-3H3,(H,30,35)(H,33,36)/t20?,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144386
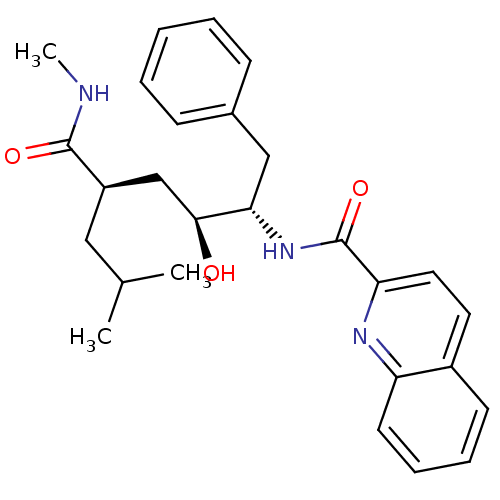
(CHEMBL69978 | Quinoline-2-carboxylic acid ((1S,2S,...)Show SMILES CNC(=O)[C@H](CC(C)C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccc2ccccc2n1 Show InChI InChI=1S/C27H33N3O3/c1-18(2)15-21(26(32)28-3)17-25(31)24(16-19-9-5-4-6-10-19)30-27(33)23-14-13-20-11-7-8-12-22(20)29-23/h4-14,18,21,24-25,31H,15-17H2,1-3H3,(H,28,32)(H,30,33)/t21-,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555221

(CHEMBL4800434) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50461352
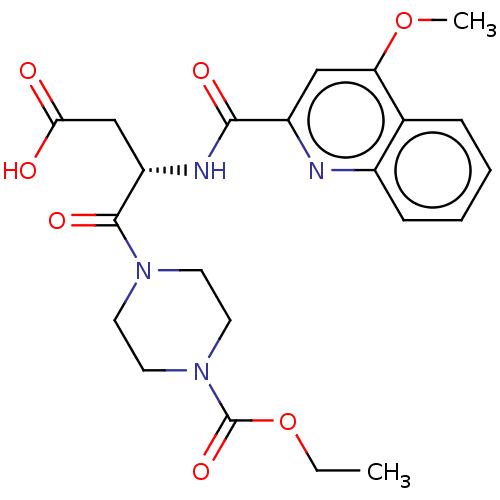
(CHEMBL4228996)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](CC(O)=O)NC(=O)c1cc(OC)c2ccccc2n1 |r| Show InChI InChI=1S/C22H26N4O7/c1-3-33-22(31)26-10-8-25(9-11-26)21(30)17(13-19(27)28)24-20(29)16-12-18(32-2)14-6-4-5-7-15(14)23-16/h4-7,12,17H,3,8-11,13H2,1-2H3,(H,24,29)(H,27,28)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdullah International Medical Research Center/King Saud bin Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]2MeSADP from P2Y12 receptor in human platelets after 60 mins by TopCount scintillation counting method |
Bioorg Med Chem Lett 28: 1459-1463 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.090
BindingDB Entry DOI: 10.7270/Q2TF010Z |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144411

(CHEMBL71242 | Quinoxaline-2-carboxylic acid [(1S,2...)Show SMILES CNC(=O)[C@H](CC(C)C)C[C@H](O)[C@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C26H30Cl2N4O3/c1-15(2)10-17(25(34)29-3)13-24(33)22(12-16-8-9-18(27)19(28)11-16)32-26(35)23-14-30-20-6-4-5-7-21(20)31-23/h4-9,11,14-15,17,22,24,33H,10,12-13H2,1-3H3,(H,29,34)(H,32,35)/t17-,22+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2169-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.021
BindingDB Entry DOI: 10.7270/Q2833RGN |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50461362
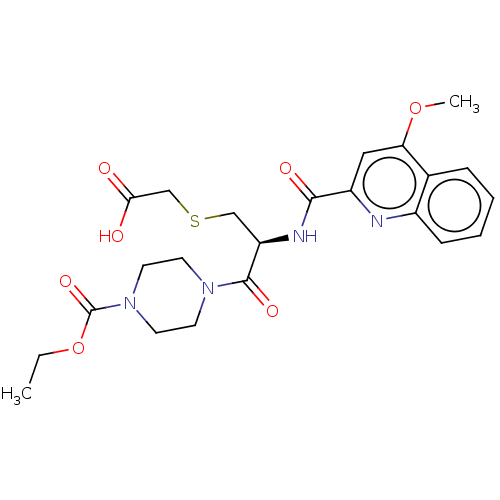
(CHEMBL4226648)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@@H](CSCC(O)=O)NC(=O)c1cc(OC)c2ccccc2n1 |r| Show InChI InChI=1S/C23H28N4O7S/c1-3-34-23(32)27-10-8-26(9-11-27)22(31)18(13-35-14-20(28)29)25-21(30)17-12-19(33-2)15-6-4-5-7-16(15)24-17/h4-7,12,18H,3,8-11,13-14H2,1-2H3,(H,25,30)(H,28,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdullah International Medical Research Center/King Saud bin Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]2MeSADP from P2Y12 receptor in human platelets after 60 mins by TopCount scintillation counting method |
Bioorg Med Chem Lett 28: 1459-1463 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.090
BindingDB Entry DOI: 10.7270/Q2TF010Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data