

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484748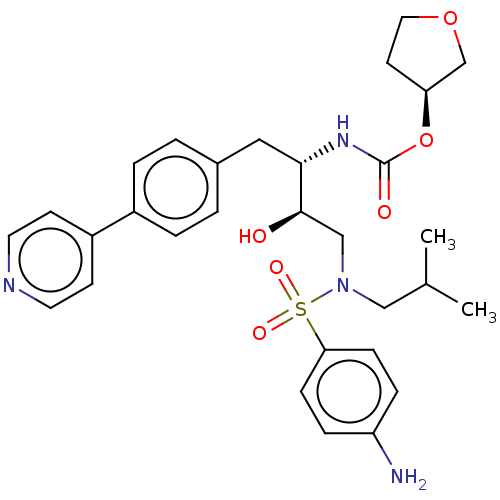 (CHEMBL1957077) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506207 (CHEMBL4466064) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506217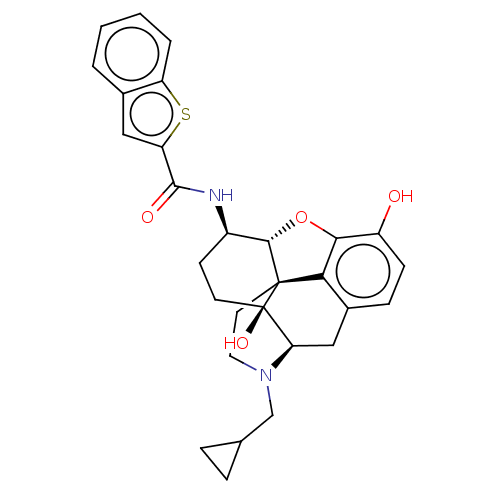 (CHEMBL4458688) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506210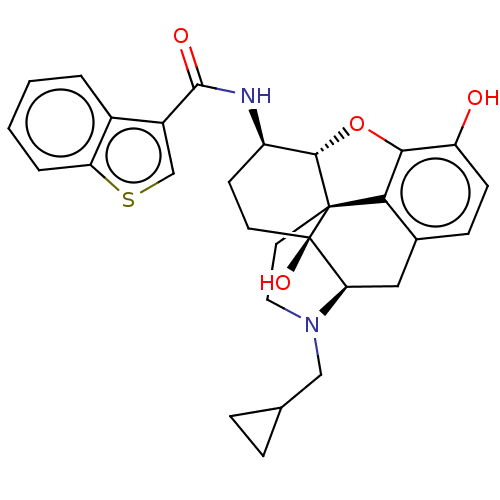 (CHEMBL4455793) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506225 (CHEMBL4562210) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506219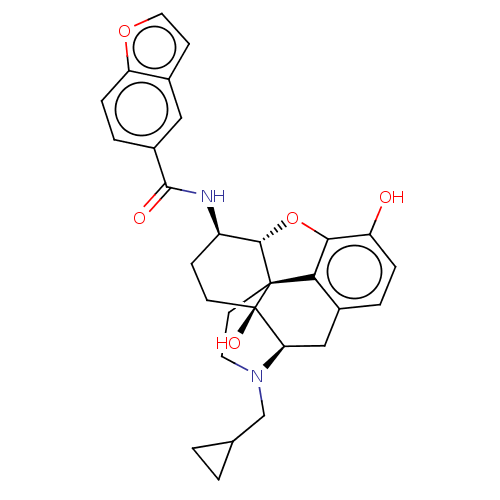 (CHEMBL4460495) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506211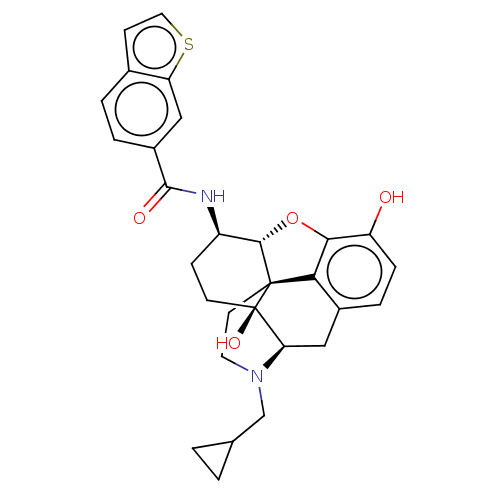 (CHEMBL4444746) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586108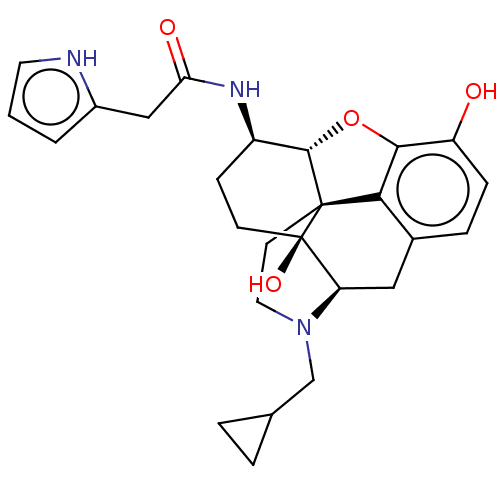 (CHEMBL5094608) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506231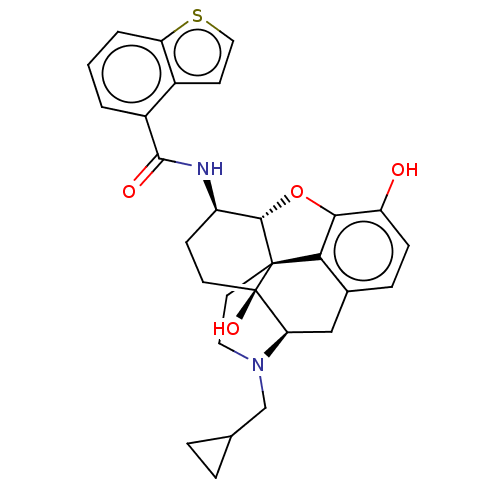 (CHEMBL4575933) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506228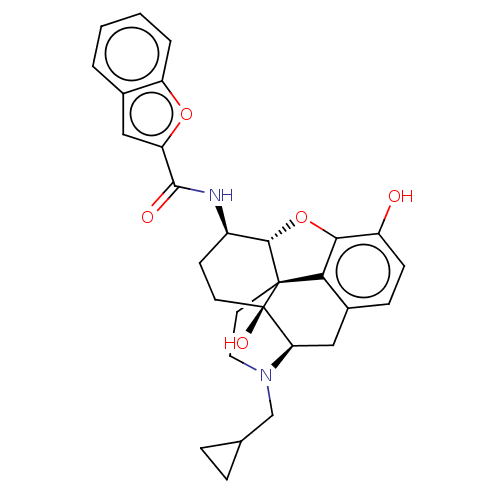 (CHEMBL4570108) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-1 (Homo sapiens (Human)) | BDBM50538006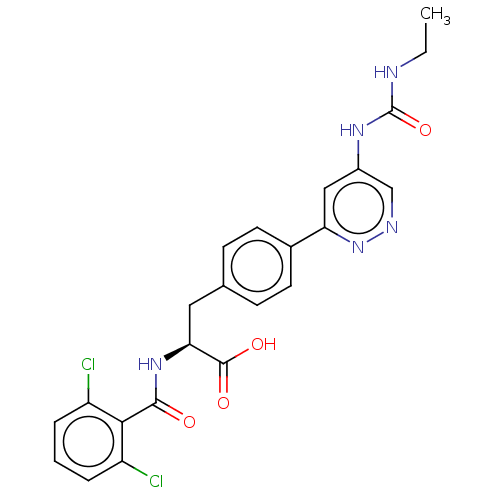 (CHEMBL4649232) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H] C8 from human integrin alphavbeta1 incubated for 6 hrs by competition binding assay | J Med Chem 63: 5675-5696 (2020) Article DOI: 10.1021/acs.jmedchem.9b01869 BindingDB Entry DOI: 10.7270/Q2DR302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50508336 (CHEMBL4530500) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cell membranes by competitive radioligand binding assay | J Med Chem 62: 561-574 (2019) Article DOI: 10.1021/acs.jmedchem.8b01158 BindingDB Entry DOI: 10.7270/Q2CN776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506218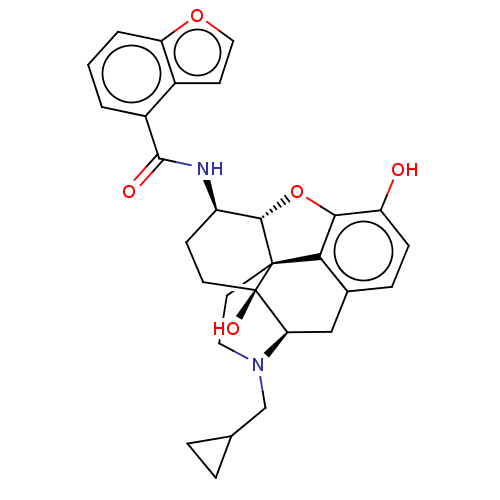 (CHEMBL4565323) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506220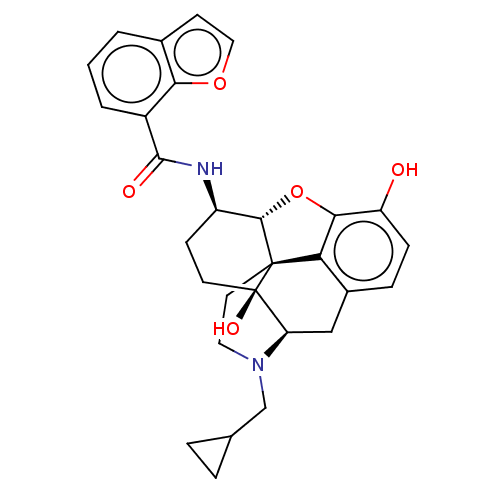 (CHEMBL4588474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506213 (CHEMBL4457013) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506208 (CHEMBL4466248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50252445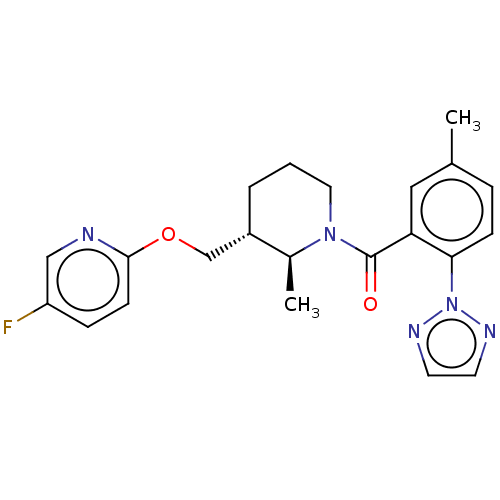 (CHEMBL4070855) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan plc, 2525 Dupont Drive, Irvine, CA 92612, USA. Electronic address: yajun.zheng@allergan.com. Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from recombinant hum... | Bioorg Med Chem Lett 27: 2825-2837 (2017) Article DOI: 10.1016/j.bmcl.2017.04.079 BindingDB Entry DOI: 10.7270/Q2TF00SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50506211 (CHEMBL4444746) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naltrindole from mouse kappa opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding ass... | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586137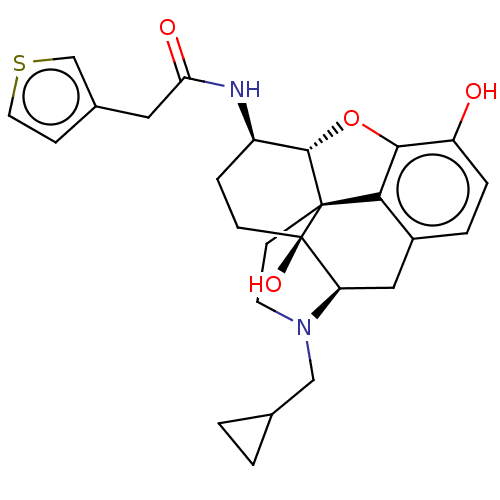 (CHEMBL5085590) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50506217 (CHEMBL4458688) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naltrindole from mouse kappa opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding ass... | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506222 (CHEMBL4572619) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50506225 (CHEMBL4562210) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naltrindole from mouse kappa opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding ass... | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586139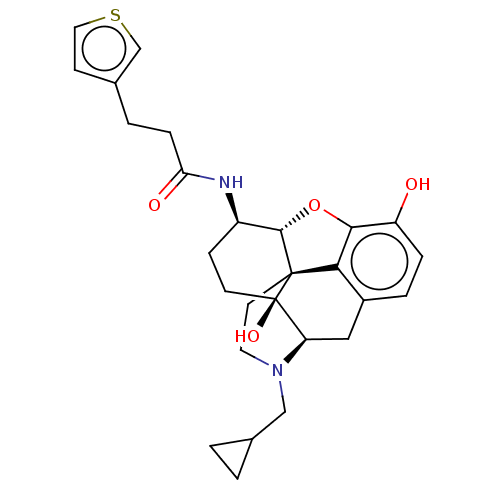 (CHEMBL5092405) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586128 (CHEMBL5089829) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50506219 (CHEMBL4460495) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naltrindole from mouse kappa opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding ass... | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50586139 (CHEMBL5092405) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-diprenorphine from mouse kappa opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586126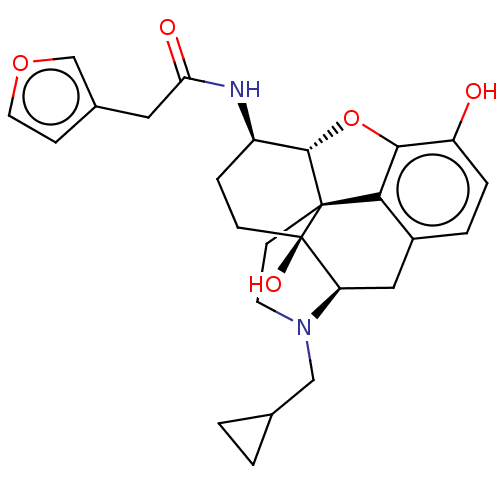 (CHEMBL5081715) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586136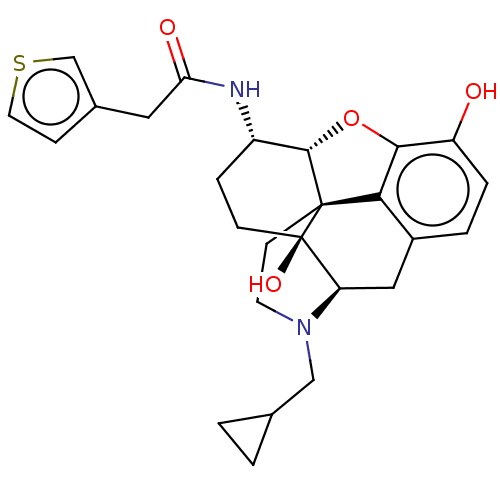 (CHEMBL5070150) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50586133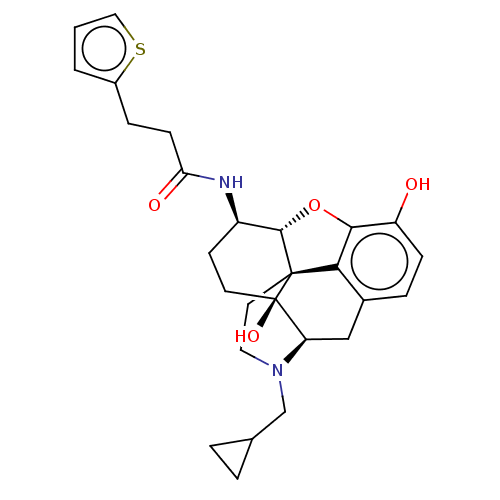 (CHEMBL5076910) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-diprenorphine from mouse kappa opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586138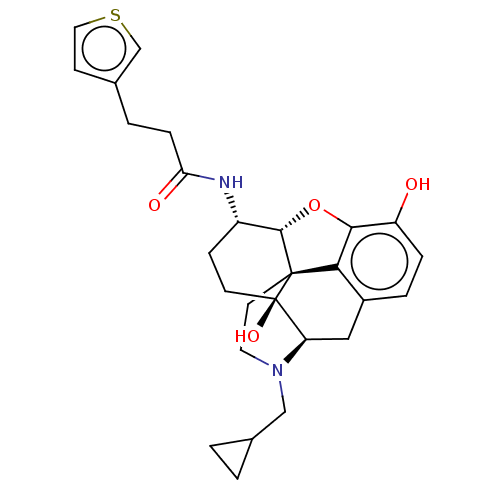 (CHEMBL5089759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50506228 (CHEMBL4570108) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naltrindole from mouse kappa opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding ass... | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506230 (CHEMBL4471389) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586120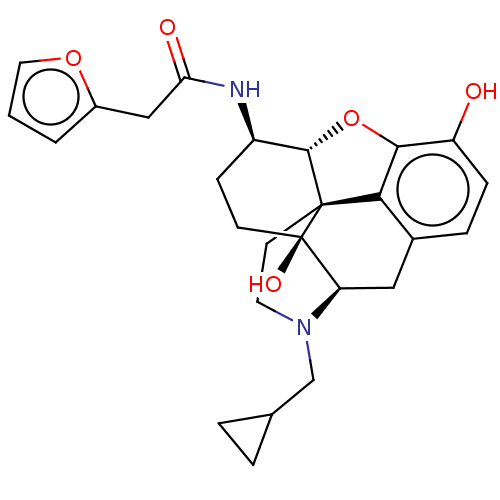 (CHEMBL5085221) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586131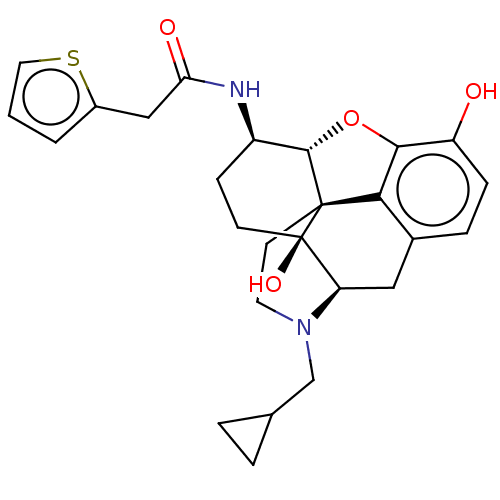 (CHEMBL5082679) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586130 (CHEMBL5075108) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586135 (CHEMBL5088056) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506214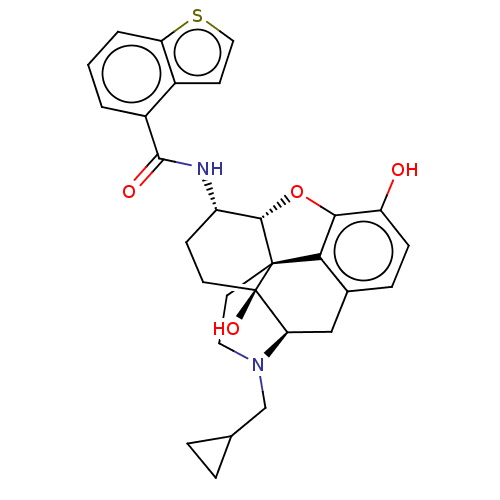 (CHEMBL4463978) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506223 (CHEMBL4476318) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586133 (CHEMBL5076910) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506226 (CHEMBL4439476) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506221 (CHEMBL4552786) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506229 (CHEMBL4552608) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506224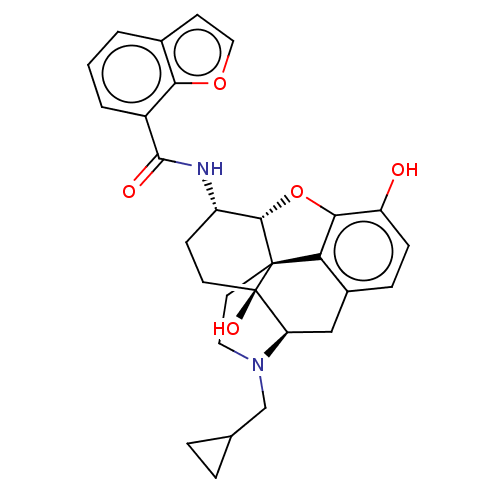 (CHEMBL4442161) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586122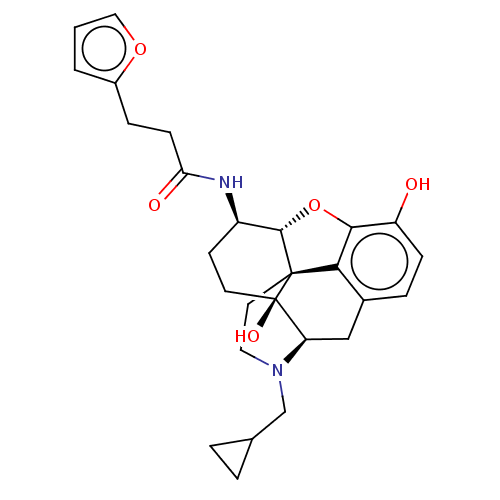 (CHEMBL5090637) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586113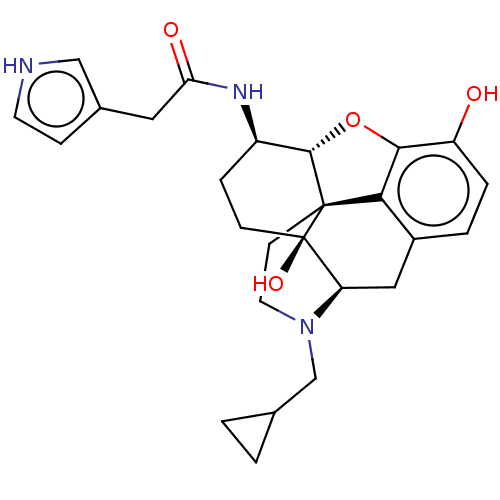 (CHEMBL5085865) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50506227 (CHEMBL4459104) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50506207 (CHEMBL4466064) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naltrindole from mouse kappa opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding ass... | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532486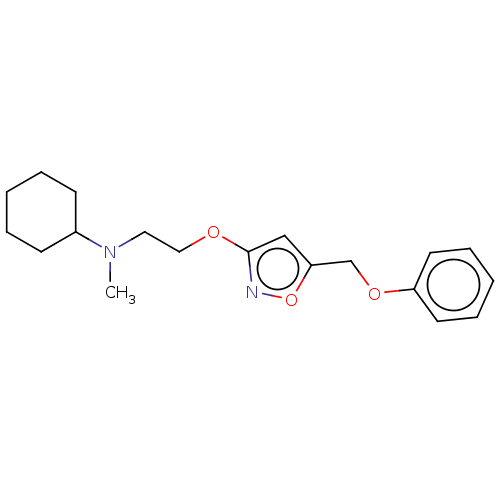 (CHEMBL4564992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50506213 (CHEMBL4457013) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-Naltrindole from mouse kappa opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding ass... | J Med Chem 62: 11399-11415 (2019) Article DOI: 10.1021/acs.jmedchem.9b01767 BindingDB Entry DOI: 10.7270/Q2G73J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50586118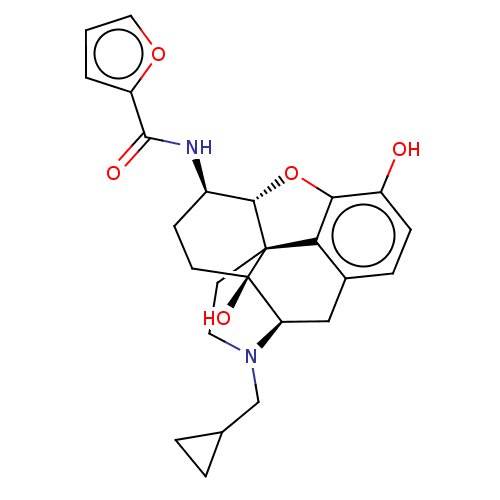 (CHEMBL5083695) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00087 BindingDB Entry DOI: 10.7270/Q21Z489F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8091 total ) | Next | Last >> |