

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484748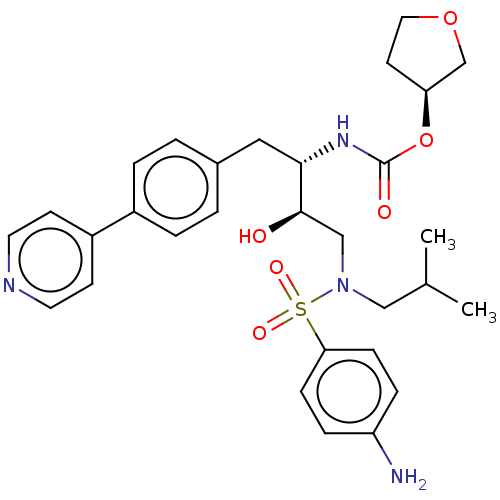 (CHEMBL1957077) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484746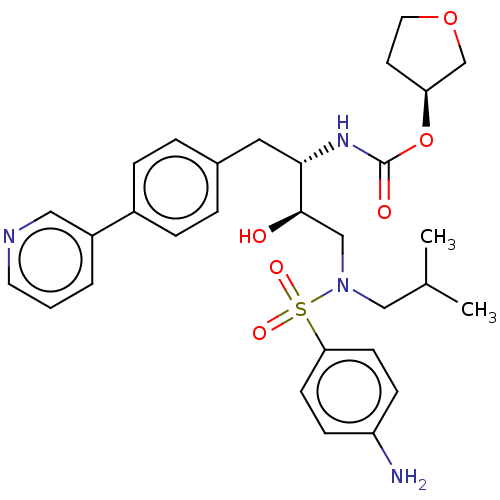 (CHEMBL1957076) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484737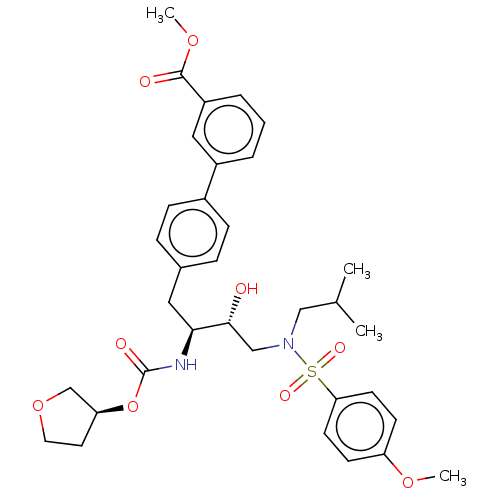 (CHEMBL1957071) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484739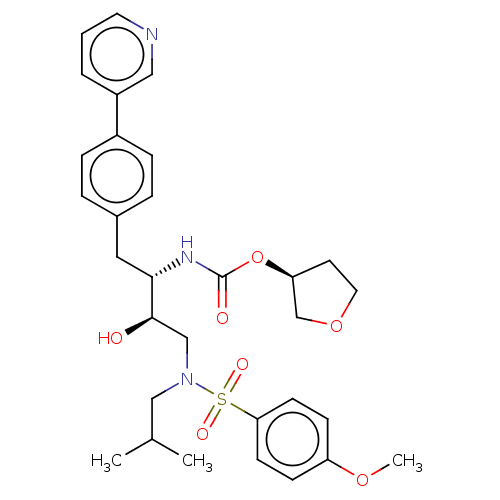 (CHEMBL1957073) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484735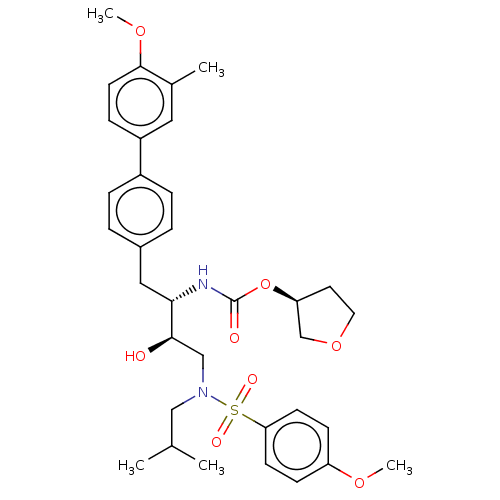 (CHEMBL1957068) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484749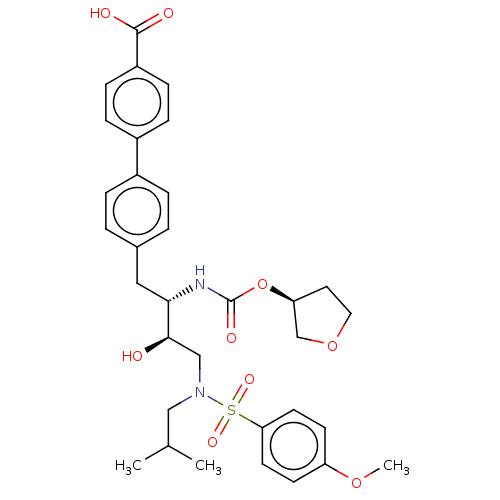 (CHEMBL1957069) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484740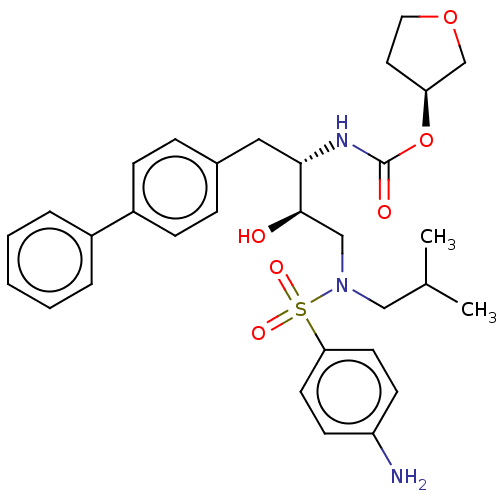 (CHEMBL1957075) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484745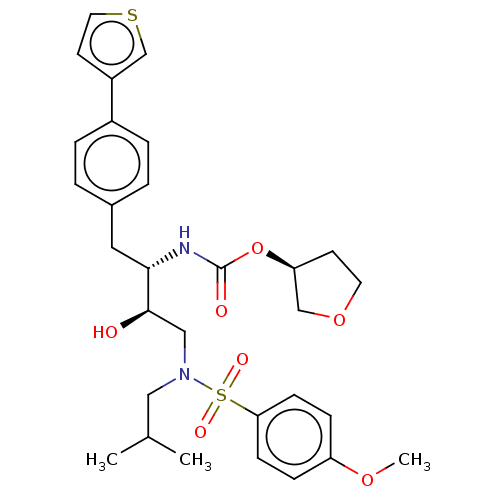 (CHEMBL1955878) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484743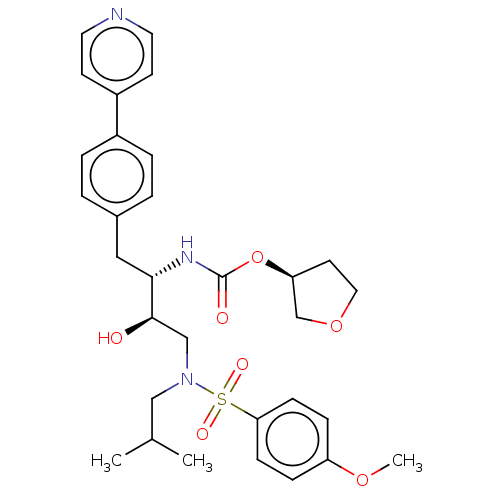 (CHEMBL1957074) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484736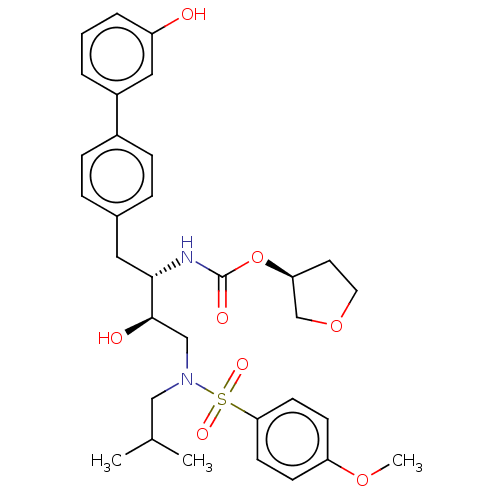 (CHEMBL1957070) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484747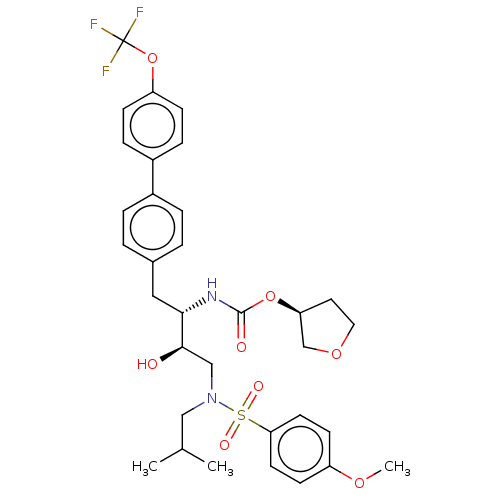 (CHEMBL1957067) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484734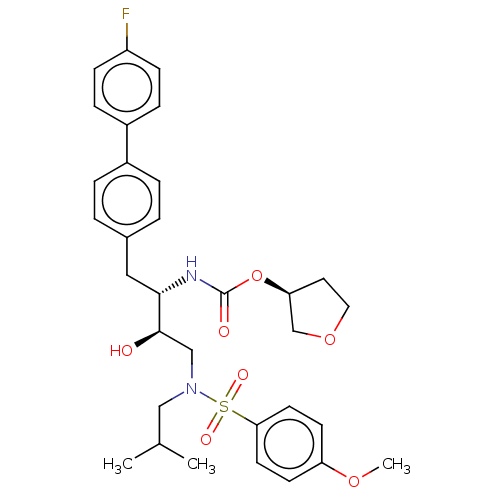 (CHEMBL1957063) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484742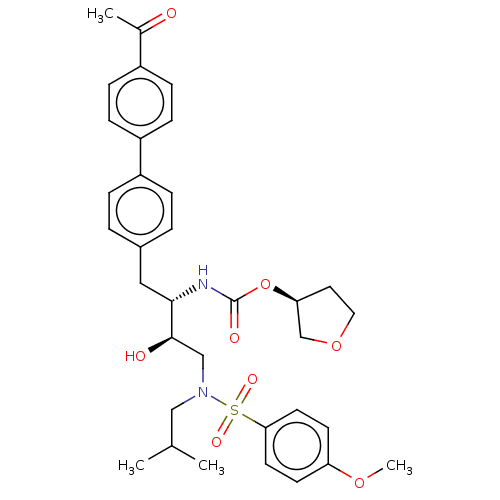 (CHEMBL1957065) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484744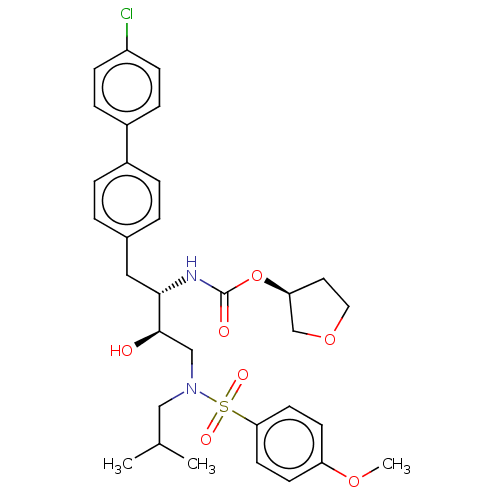 (CHEMBL1957064) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484741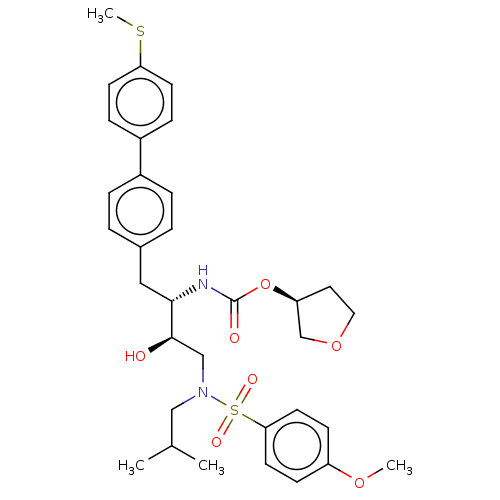 (CHEMBL1957066) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484738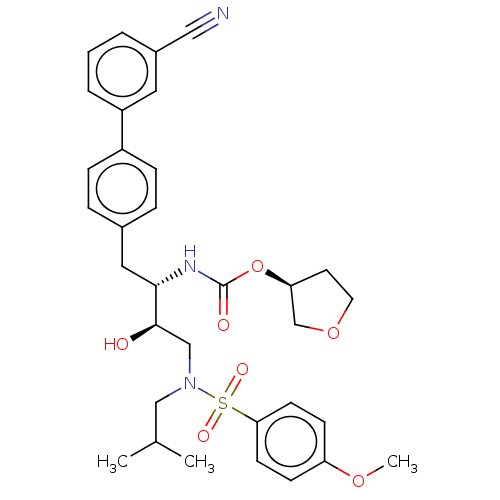 (CHEMBL1957072) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485688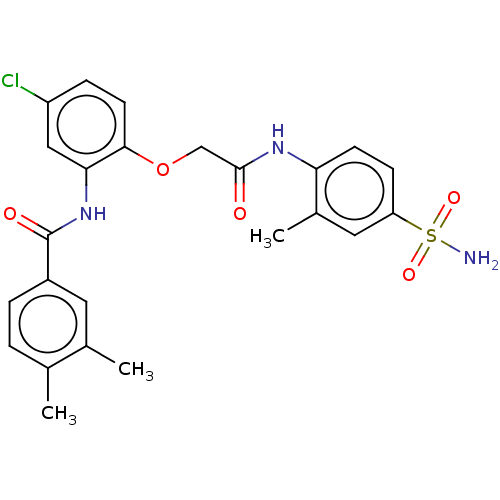 (CHEMBL2151836) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485689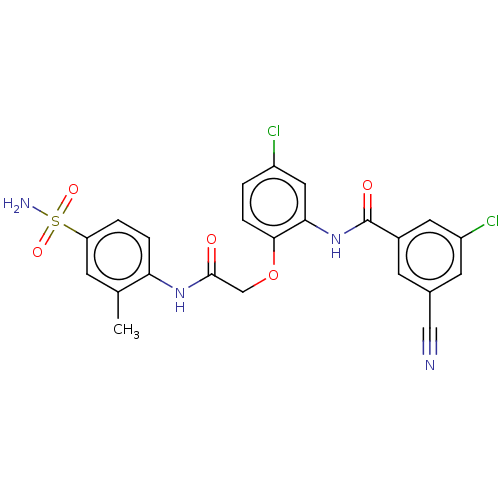 (CHEMBL2151844) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464161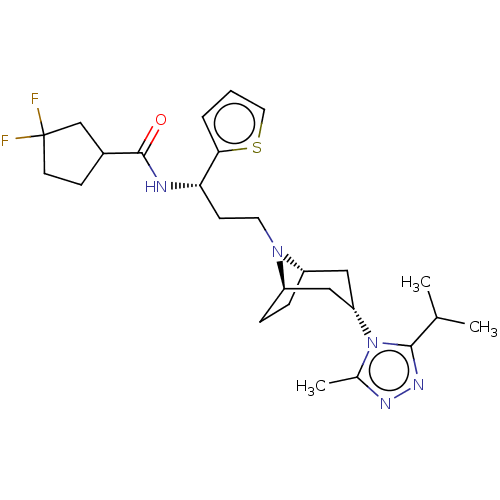 (CHEMBL4248009) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464149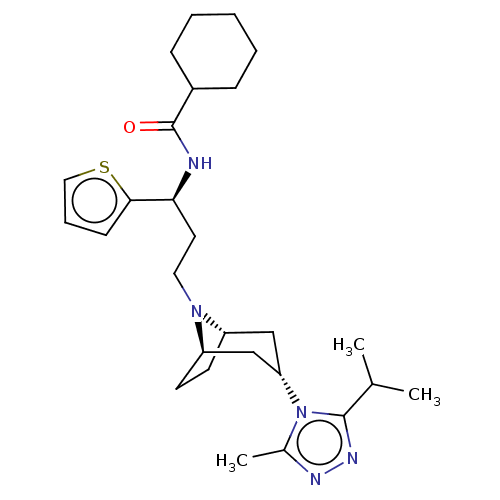 (CHEMBL4242114) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50602353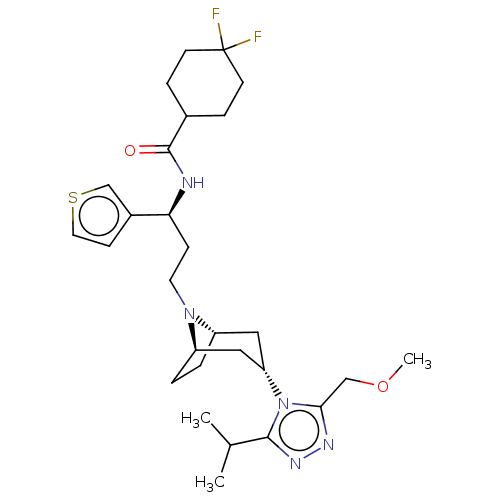 (CHEMBL5171331) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01383 BindingDB Entry DOI: 10.7270/Q2CZ3C7H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464159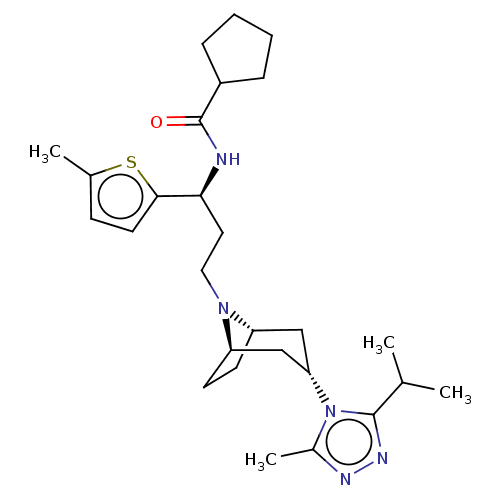 (CHEMBL4242489) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464166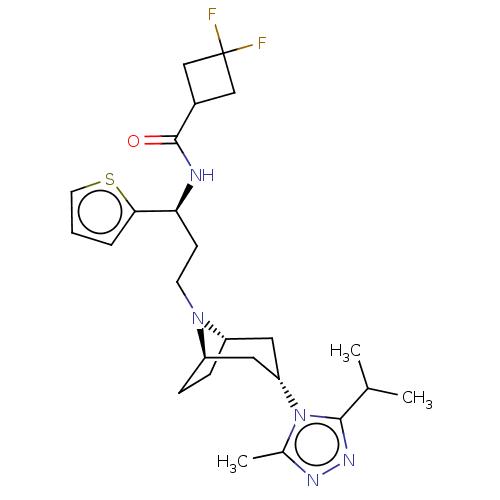 (CHEMBL4248472) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464162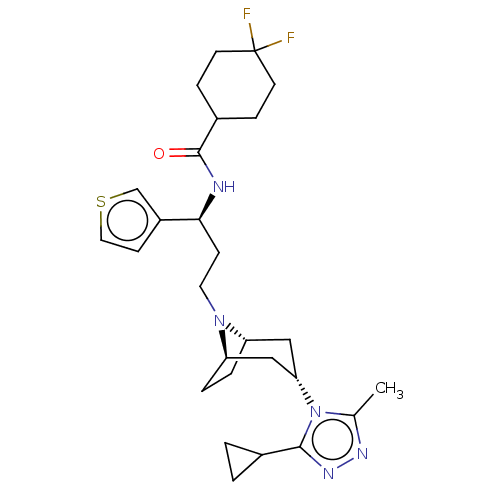 (CHEMBL4242913) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478026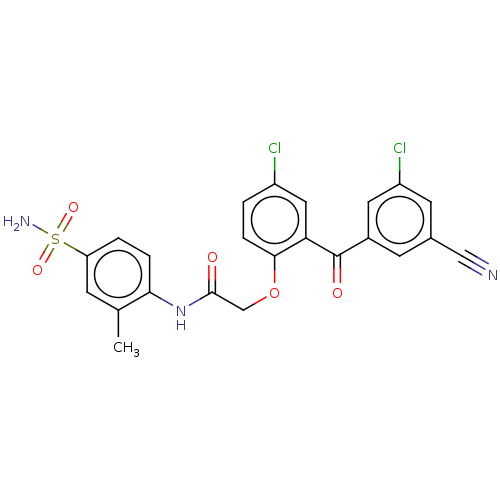 (CHEMBL203420 | GW678248 | GW8248) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464142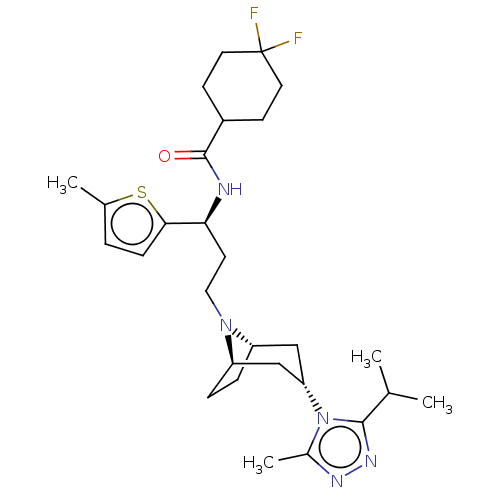 (CHEMBL4248282) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464163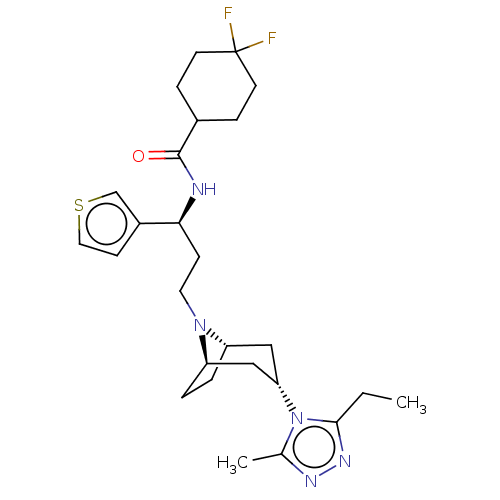 (CHEMBL4240475) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464165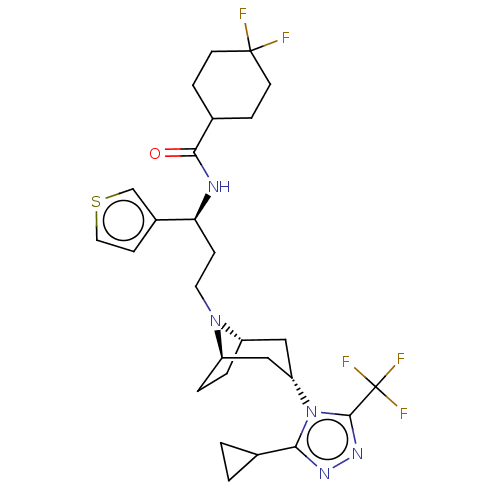 (CHEMBL4239790) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464160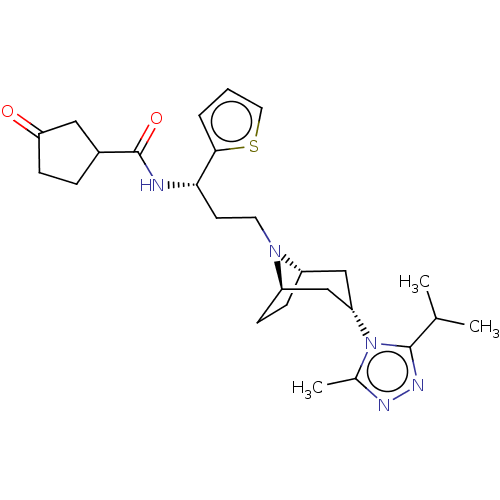 (CHEMBL4241721) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464169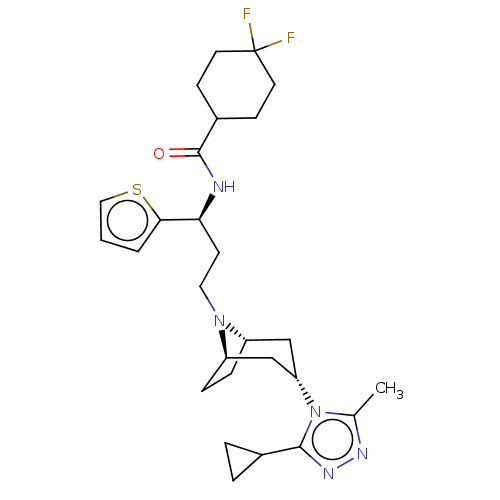 (CHEMBL4244672) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464168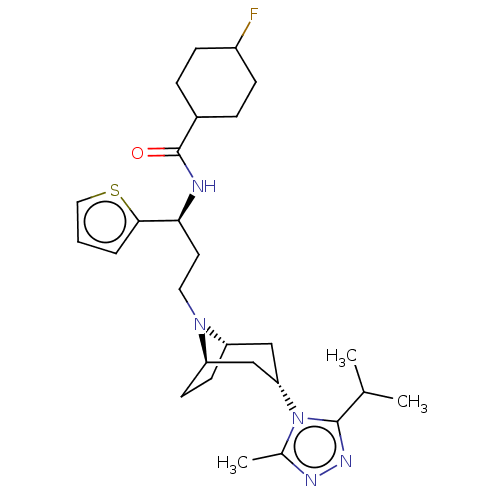 (CHEMBL4237964) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464136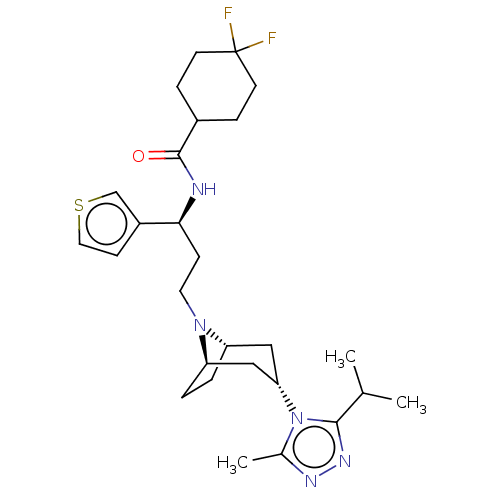 (CHEMBL4249798) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464140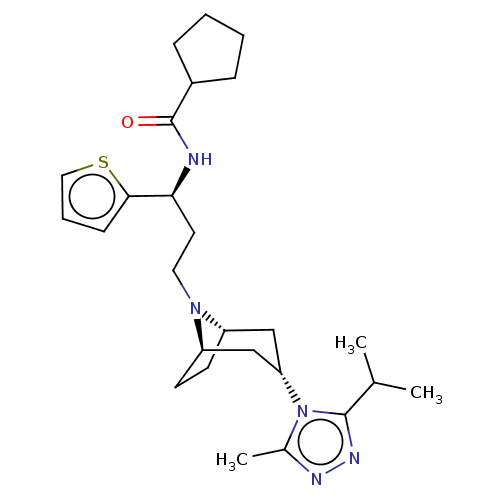 (CHEMBL4238063) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464146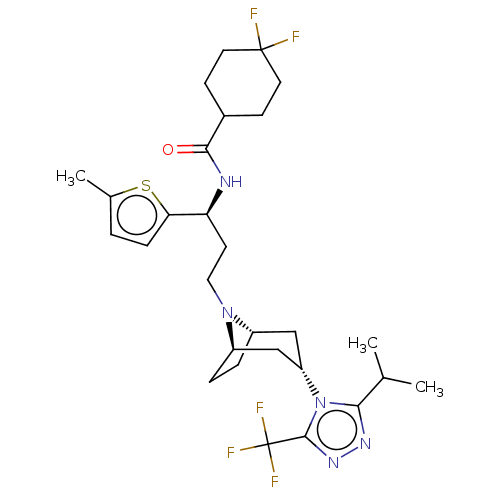 (CHEMBL4250366) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50602350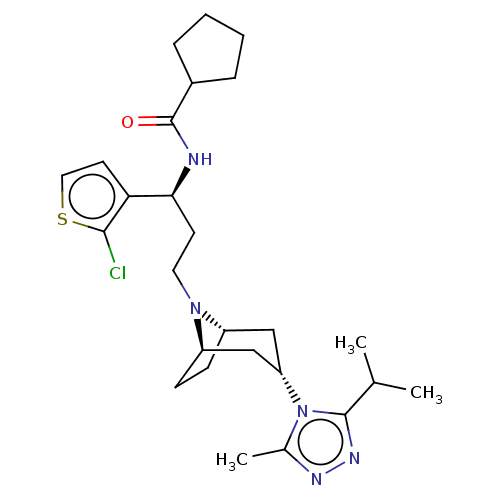 (CHEMBL5180628) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01383 BindingDB Entry DOI: 10.7270/Q2CZ3C7H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464164 (CHEMBL4240242) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464139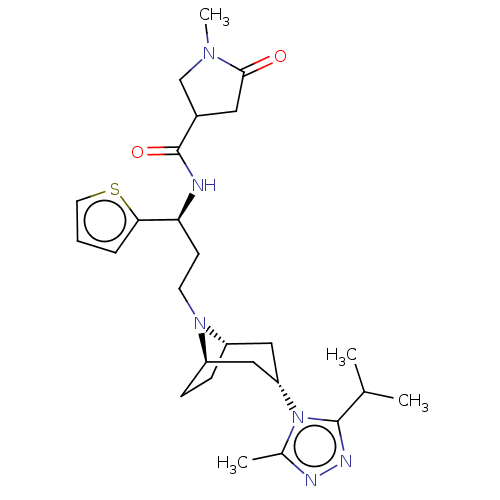 (CHEMBL4238007) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50602351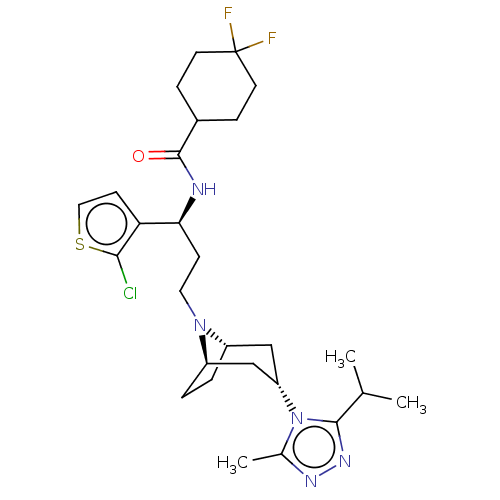 (CHEMBL5177519) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01383 BindingDB Entry DOI: 10.7270/Q2CZ3C7H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464170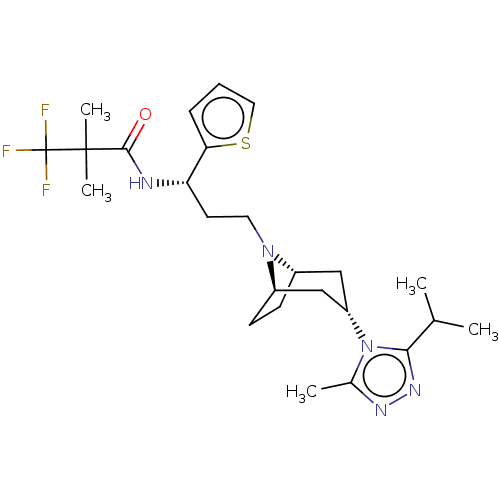 (CHEMBL4241284) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464156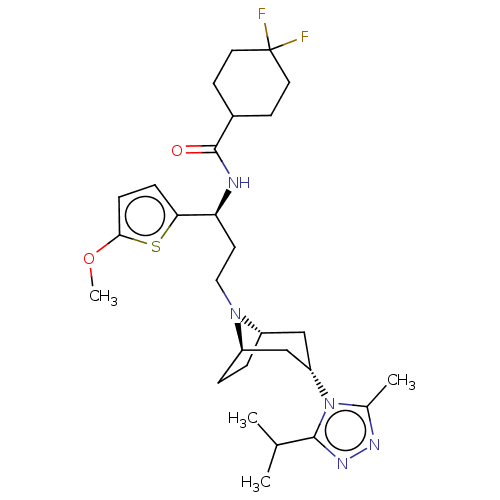 (CHEMBL4249941) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464148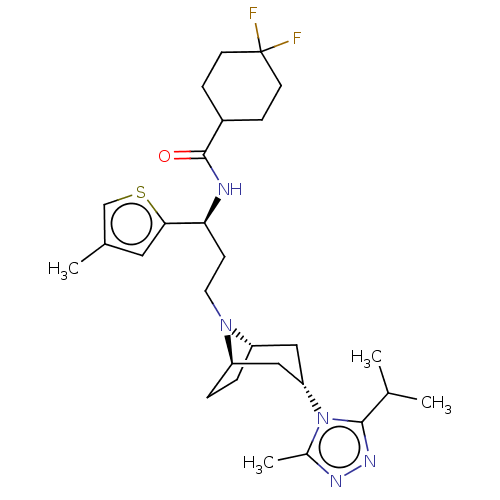 (CHEMBL4237581) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448308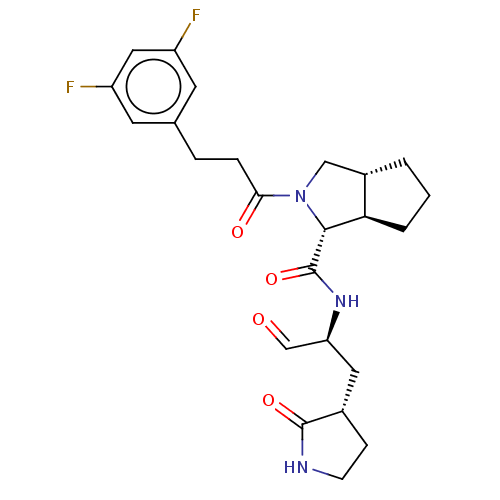 (MI-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448306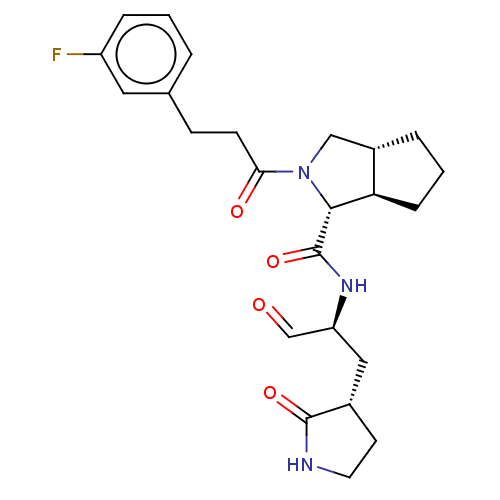 (MI-21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50602343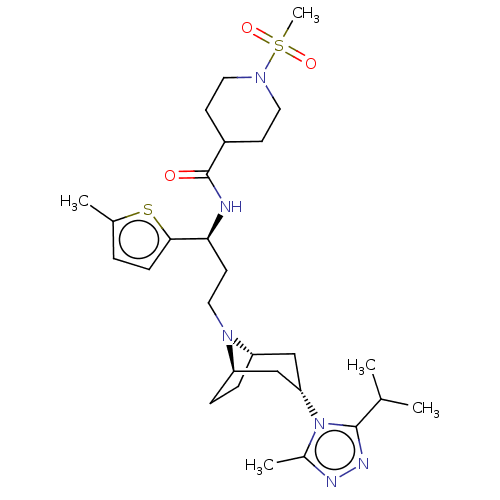 (CHEMBL5205216) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01383 BindingDB Entry DOI: 10.7270/Q2CZ3C7H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334986 (4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01383 BindingDB Entry DOI: 10.7270/Q2CZ3C7H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464147 (CHEMBL256907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464155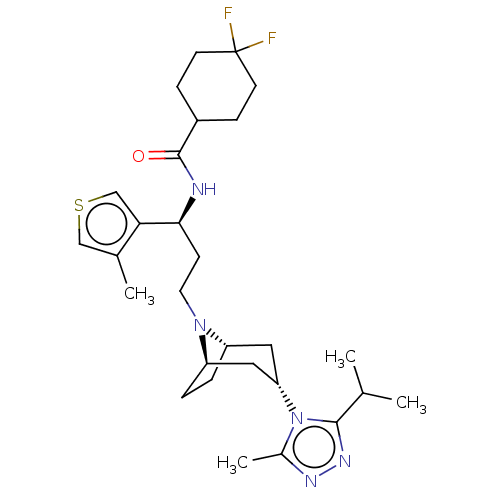 (CHEMBL4241066) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50602354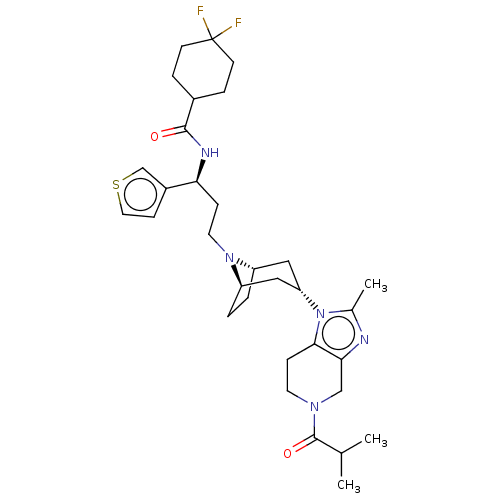 (CHEMBL5209067) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01383 BindingDB Entry DOI: 10.7270/Q2CZ3C7H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464141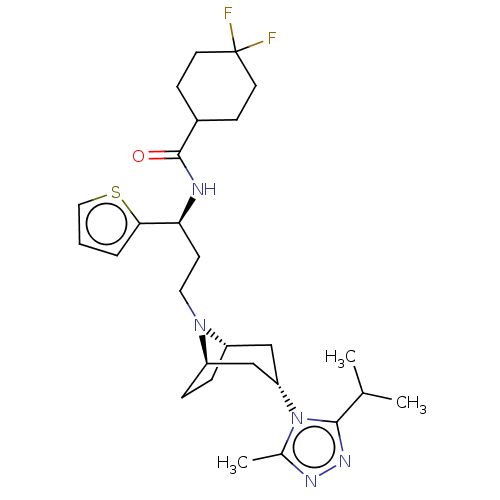 (CHEMBL4244935) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... | J Med Chem 61: 9621-9636 (2018) Article DOI: 10.1021/acs.jmedchem.8b01077 BindingDB Entry DOI: 10.7270/Q2W95CVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 467 total ) | Next | Last >> |