

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581953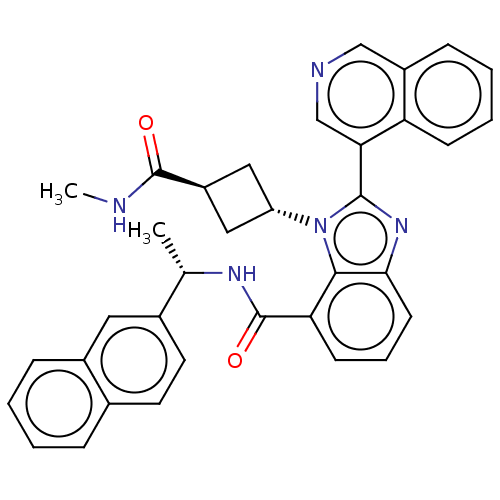 (WO2023004291, Compound CDD-1819) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem | WIPO WO2023004291 | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581952 (WO2023004291, Compound CDD-1830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581948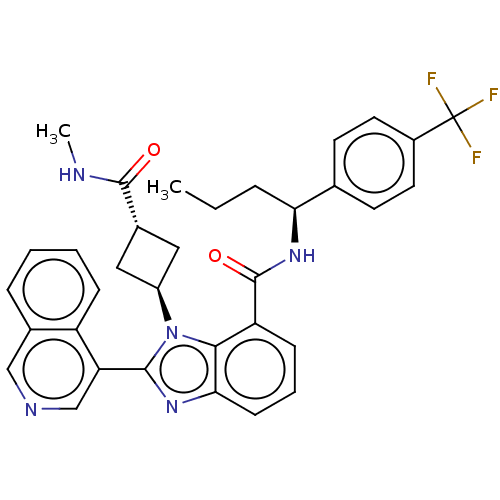 (WO2023004291, Compound CDD-1733) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem | WIPO WO2023004291 | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581950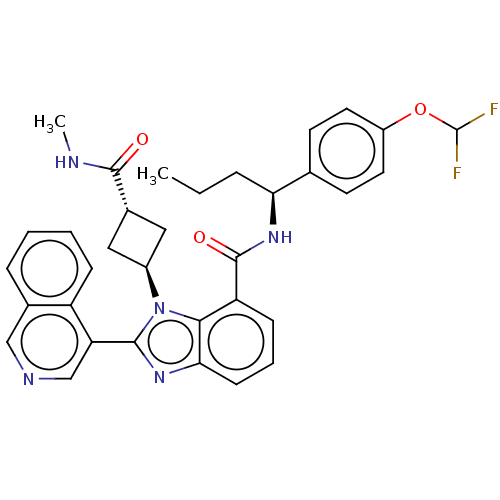 (WO2023004291, Compound CDD-1780) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab-His6 (2019-nCoV) | BDBM581944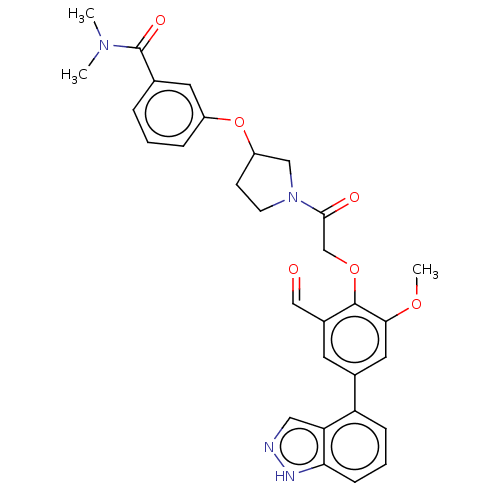 (WO2023004291, Compound CDD-1714) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581944 (WO2023004291, Compound CDD-1714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581951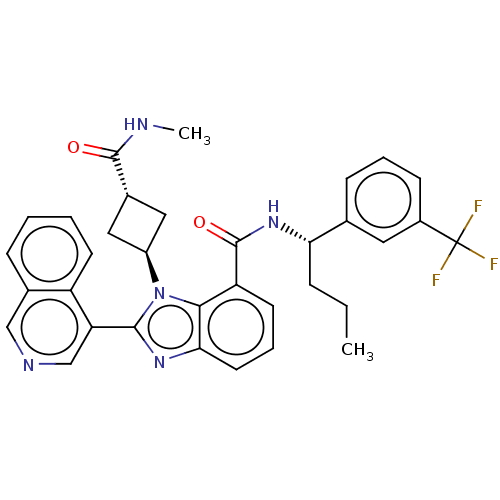 (WO2023004291, Compound CDD-1795) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab-His6 (2019-nCoV) | BDBM484197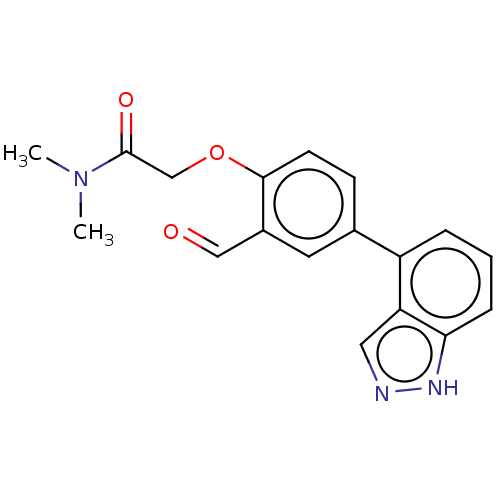 (CDD-1976 | WO2023004291, Compound CDD-1976) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50407504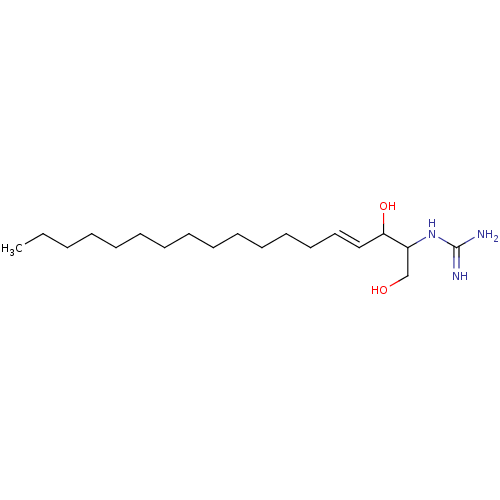 (CHEMBL5275740) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat prostatic vas deferens (Alpha-1A adrenergic receptor ) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484197 (CDD-1976 | WO2023004291, Compound CDD-1976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab-His6 (2019-nCoV) | BDBM484196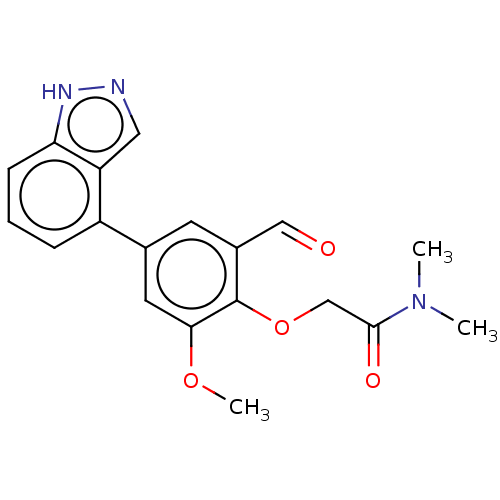 (CDD-1713 | WO2023004291, Compound CDD-1713) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | WIPO WO2023004291 | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581949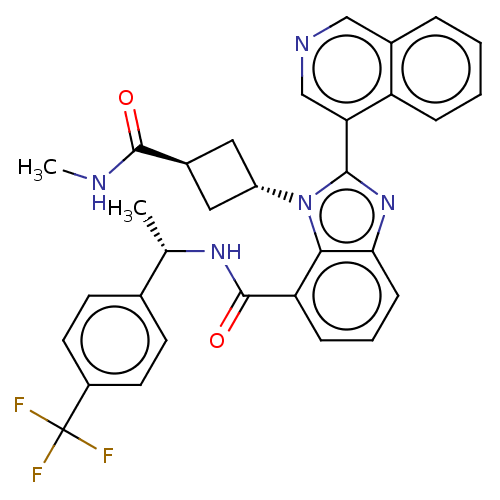 (WO2023004291, Compound CDD-1829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484196 (CDD-1713 | WO2023004291, Compound CDD-1713) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | WIPO WO2023004291 | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50407504 (CHEMBL5275740) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of the cAMP-stimulated beta-galactosidase transcription in SK-N-MC cells expressing the human Histamine H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50600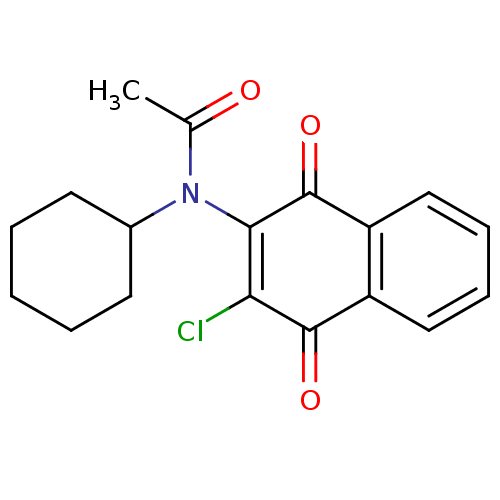 (MLS001029919 | N-(3-Chloro-1,4-dioxo-1,4-dihydro-n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Mus musculus (Mouse)) | BDBM50407506 (CHEMBL4595333) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Mus musculus (Mouse)) | BDBM50407507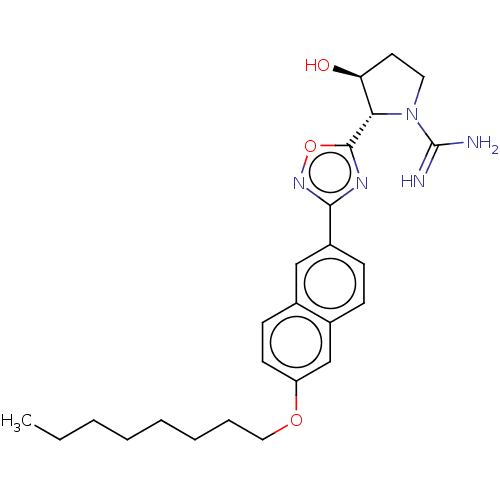 (CHEMBL5281819) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50443388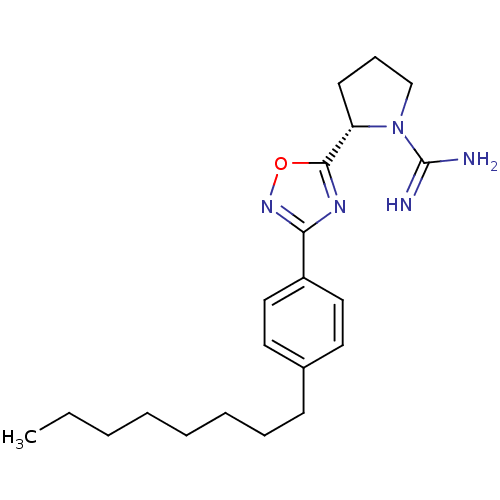 (CHEMBL3086782 | US9688668, SLR080811) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50045060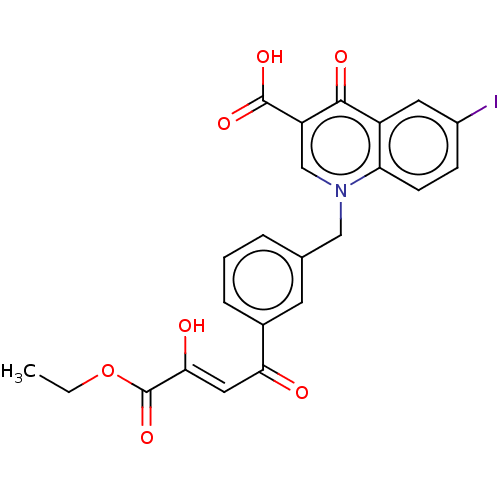 (CHEMBL3309789) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of PTP1B (unknown origin) using pNPP as substrate by Lineweaver-Burk plot | Bioorg Med Chem 22: 3670-83 (2014) Article DOI: 10.1016/j.bmc.2014.05.028 BindingDB Entry DOI: 10.7270/Q2G44RX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50009730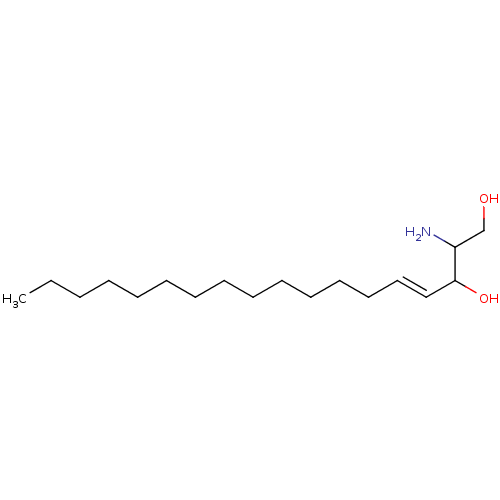 ((E)-2-Amino-octadec-4-ene-1,3-diol | 2-Amino-octad...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat prostatic vas deferens (alpha1A receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50407505 (CHEMBL447685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-phenylephrine-induced contraction of rat spleen (Alpha-1B adrenergic receptor ) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50139649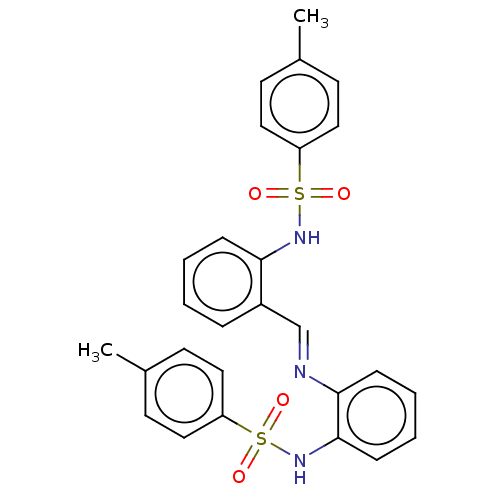 (CHEMBL3764617) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50393642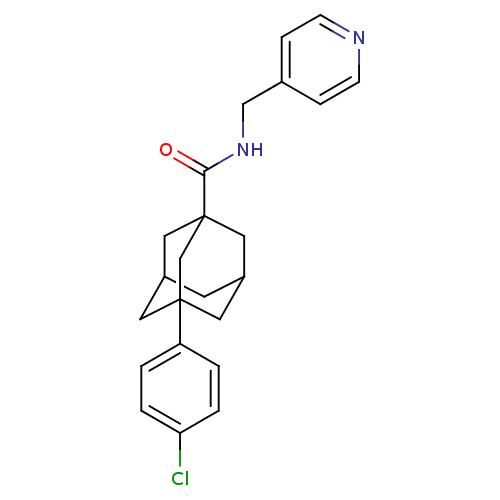 (CHEMBL2158685) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50443388 (CHEMBL3086782 | US9688668, SLR080811) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50407505 (CHEMBL447685) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab-His6 (2019-nCoV) | BDBM581945 (WO2023004291, Compound CDD-1886) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Mus musculus) | BDBM50407506 (CHEMBL4595333) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Mus musculus) | BDBM50407507 (CHEMBL5281819) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581945 (WO2023004291, Compound CDD-1886) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2023004291 | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BV7MGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50139649 (CHEMBL3764617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50021574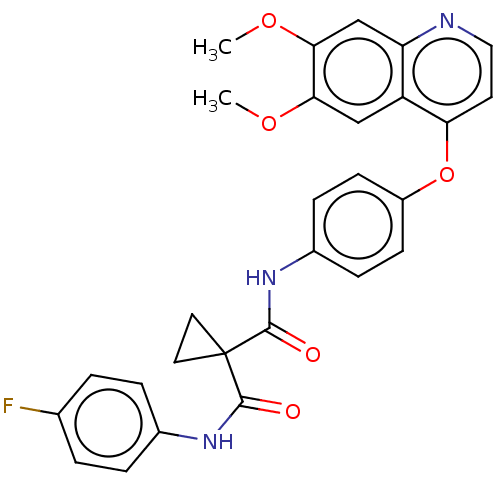 (BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length VEGFR2 using poly (Glu, Tyr) as substrate by alpha screen assay | Eur J Med Chem 138: 942-951 (2017) Article DOI: 10.1016/j.ejmech.2017.06.057 BindingDB Entry DOI: 10.7270/Q2MK6GDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270331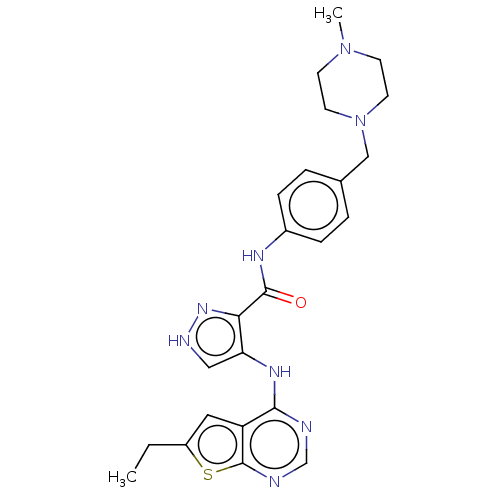 (CHEMBL4086149) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.101 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270307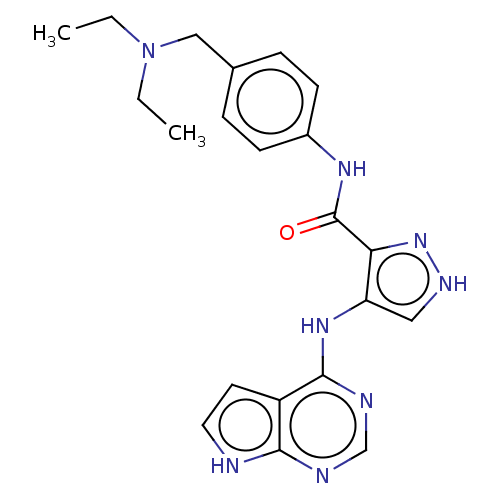 (CHEMBL4092761) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270330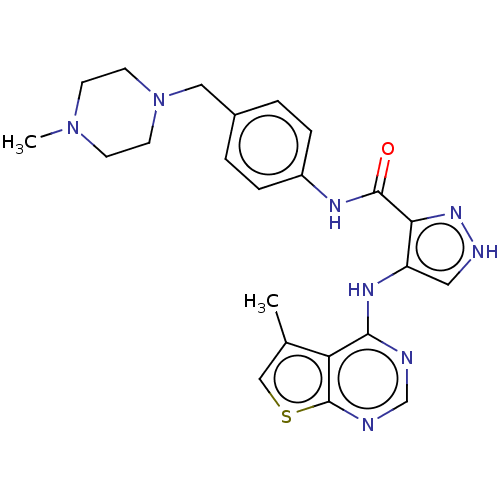 (CHEMBL4078261) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.167 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50449547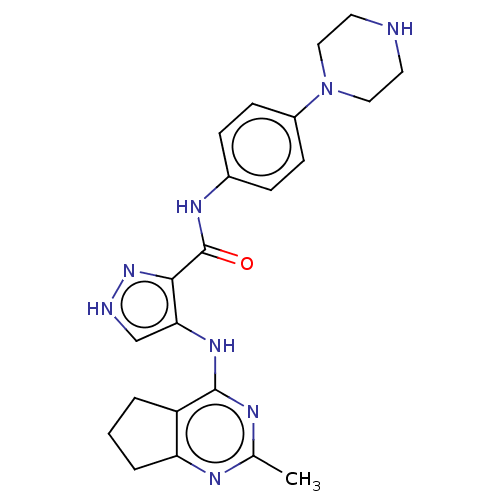 (CHEMBL4163870) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 by Hotspot assay | Eur J Med Chem 155: 303-315 (2018) Article DOI: 10.1016/j.ejmech.2018.06.010 BindingDB Entry DOI: 10.7270/Q2668GSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270304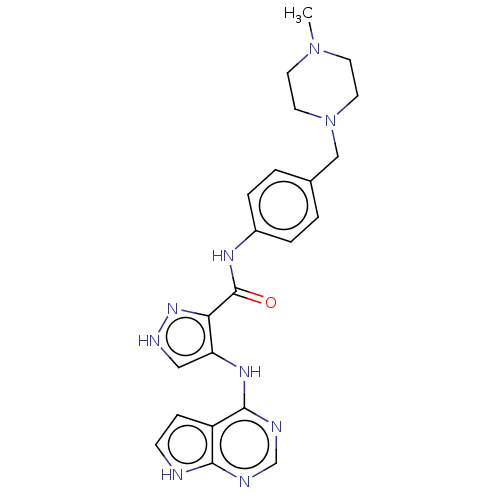 (CHEMBL4077071) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270309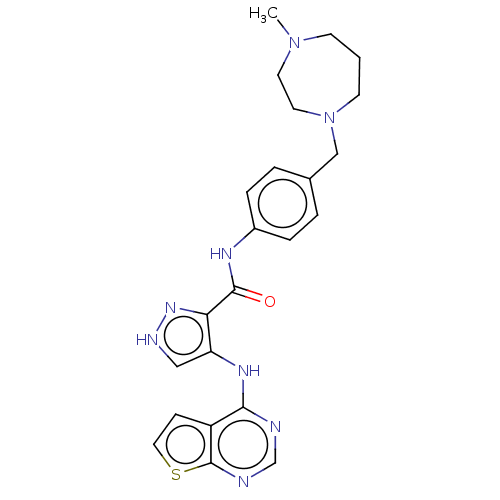 (CHEMBL4101635) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513250 (CHEMBL4475689) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.327 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270316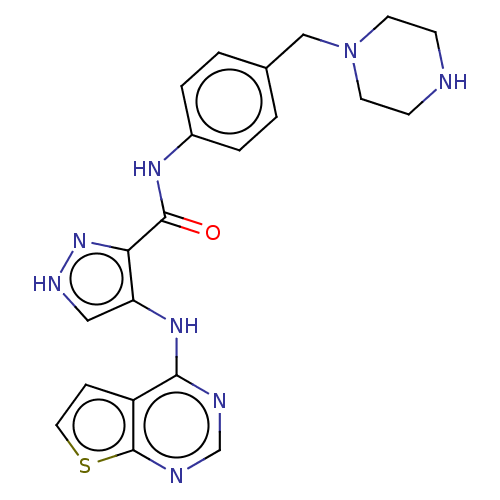 (CHEMBL4104057) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270329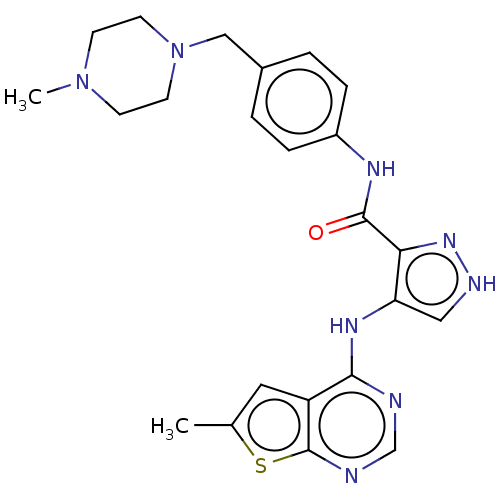 (CHEMBL4073053) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.384 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270298 (CHEMBL4075720) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.438 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50449554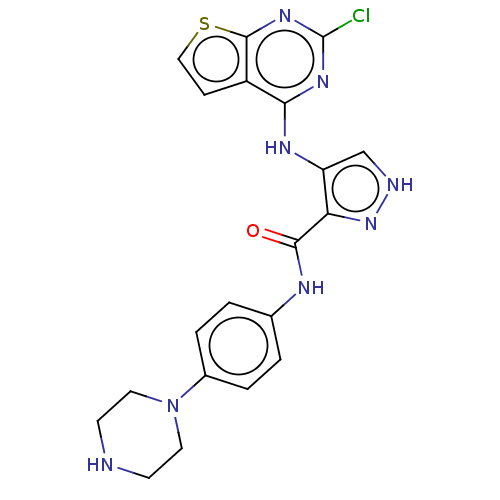 (CHEMBL4172223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 by Hotspot assay | Eur J Med Chem 155: 303-315 (2018) Article DOI: 10.1016/j.ejmech.2018.06.010 BindingDB Entry DOI: 10.7270/Q2668GSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270303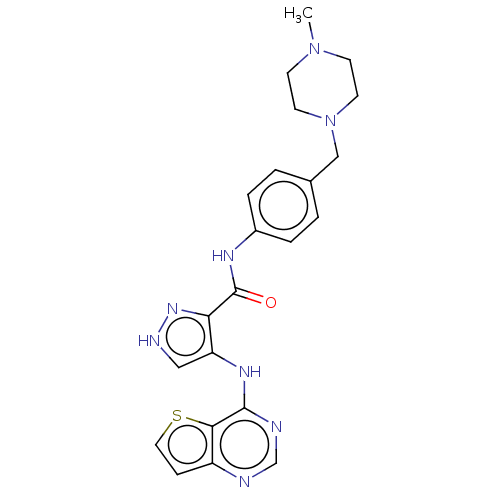 (CHEMBL4061686) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270298 (CHEMBL4075720) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 D835Y mutant by Hotspot assay | Eur J Med Chem 155: 303-315 (2018) Article DOI: 10.1016/j.ejmech.2018.06.010 BindingDB Entry DOI: 10.7270/Q2668GSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270319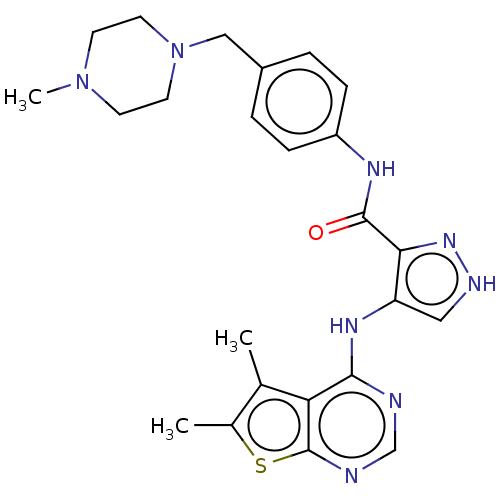 (CHEMBL4093755) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.576 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270335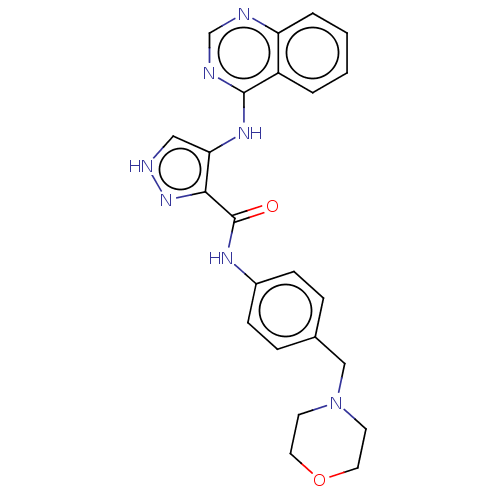 (CHEMBL4088753) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.588 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition measure after 120 mins by filt... | J Med Chem 61: 1499-1518 (2018) Article DOI: 10.1021/acs.jmedchem.7b01261 BindingDB Entry DOI: 10.7270/Q2FR003K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50270298 (CHEMBL4075720) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 by Hotspot assay | Eur J Med Chem 155: 303-315 (2018) Article DOI: 10.1016/j.ejmech.2018.06.010 BindingDB Entry DOI: 10.7270/Q2668GSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50449553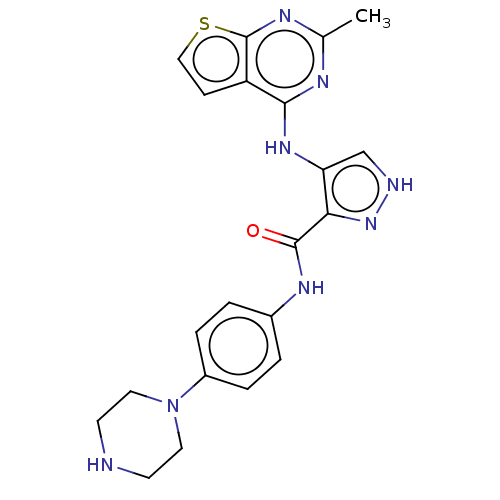 (CHEMBL4164331) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 by Hotspot assay | Eur J Med Chem 155: 303-315 (2018) Article DOI: 10.1016/j.ejmech.2018.06.010 BindingDB Entry DOI: 10.7270/Q2668GSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 605 total ) | Next | Last >> |