

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostacyclin receptor (RAT) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235370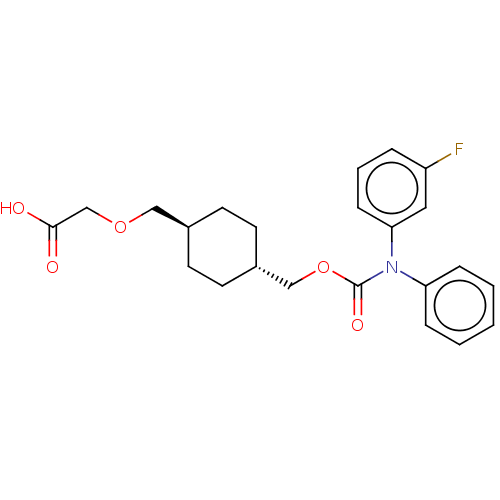 (CHEMBL3933704) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (RAT) | BDBM50235370 (CHEMBL3933704) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (RAT) | BDBM50235385 (APD-811 | Ralinepag) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50235370 (CHEMBL3933704) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235380 (CHEMBL3917503) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50235370 (CHEMBL3933704) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-PGE2 from recombinant human EP2 receptor expressed in HEK293 cell membranes incubated for 1 hr | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 678 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50235370 (CHEMBL3933704) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50235370 (CHEMBL3933704) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from recombinant human DP1 receptor | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from recombinant human DP1 receptor | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117016 (US8664258, 32 | US9987252, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125]DOI as radioligand. To define nonspecific binding, 10... | US Patent US8664258 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117016 (US8664258, 32 | US9987252, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia at Charlottesville | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | Chem Biol 14: 1186-97 (2007) BindingDB Entry DOI: 10.7270/Q29K4DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117019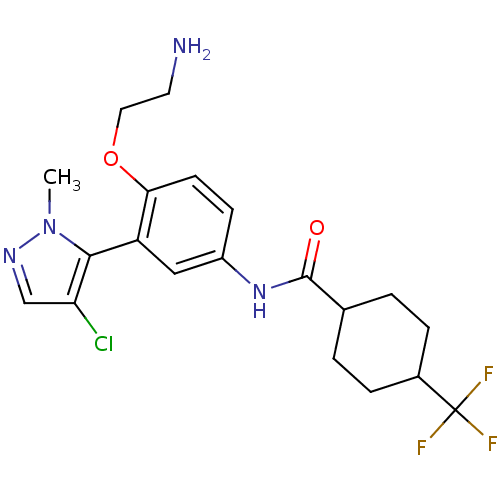 (US8664258, 303 | US9987252, 303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia at Charlottesville | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | Chem Biol 14: 1186-97 (2007) BindingDB Entry DOI: 10.7270/Q29K4DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117019 (US8664258, 303 | US9987252, 303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125]DOI as radioligand. To define nonspecific binding, 10... | US Patent US8664258 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117018 (US8664258, 197 | US9987252, 197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia at Charlottesville | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | Chem Biol 14: 1186-97 (2007) BindingDB Entry DOI: 10.7270/Q29K4DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117018 (US8664258, 197 | US9987252, 197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125]DOI as radioligand. To define nonspecific binding, 10... | US Patent US8664258 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM117020 (US8664258, 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125]DOI as radioligand. To define nonspecific binding, 10... | US Patent US8664258 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM397088 (4-Bromo-thiophene-2- carboxylic acid [4-(2- amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia at Charlottesville | Assay Description Radioligand binding assays for human 5-HT2A receptor was conducted using the 5-HT2 agonist [125I]DOI as radioligand. To define nonspecific binding, 1... | Chem Biol 14: 1186-97 (2007) BindingDB Entry DOI: 10.7270/Q29K4DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302559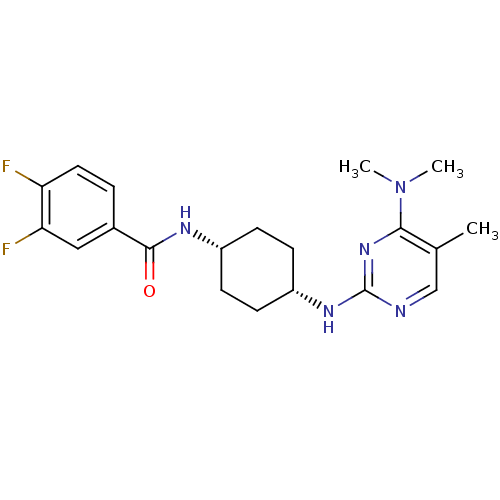 (CHEMBL578049 | N-(cis-4-{[4-(Dimethylamino)-5-meth...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302561 (CHEMBL568208 | N-((cis)-4-(4-(dimethylamino)-5,6-d...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302552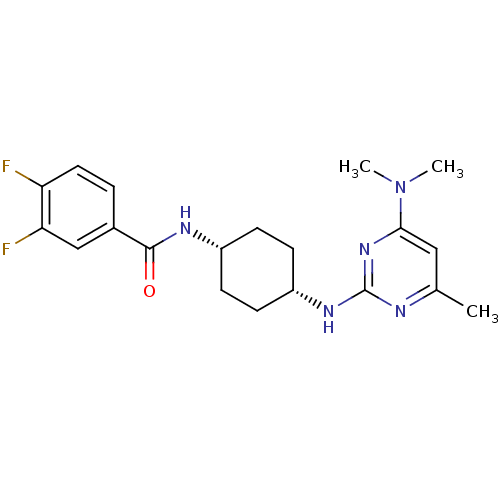 (CHEMBL578166 | N-((cis)-4-(4-(dimethylamino)-6-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302555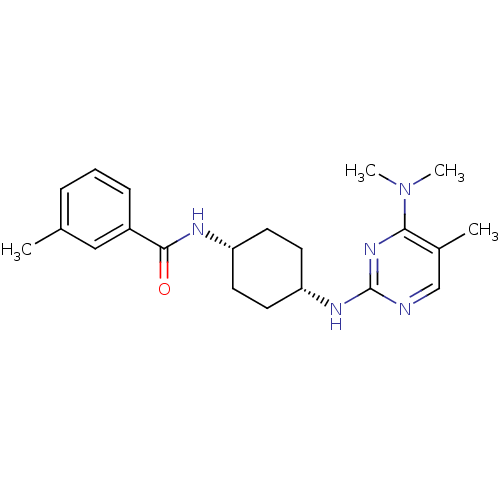 (CHEMBL567993 | N-((cis)-4-(4-(dimethylamino)-5-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302569 (3,5-dichloro-N-(((cis)-4-(4-(dimethylamino)-5-meth...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302553 (3,4-dichloro-N-((cis)-4-(4-(dimethylamino)-6-methy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302568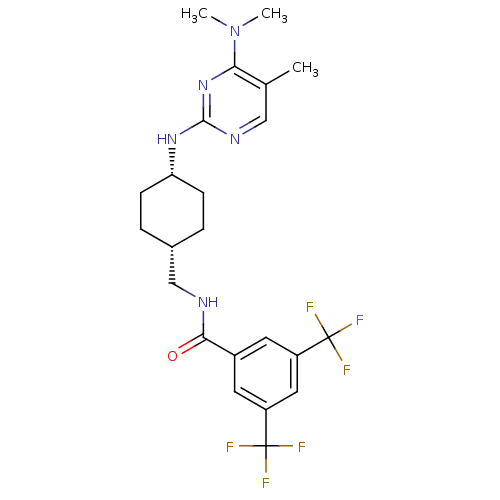 (CHEMBL566075 | N-[(cis-4-{[4-(Dimethylamino)-5-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50150715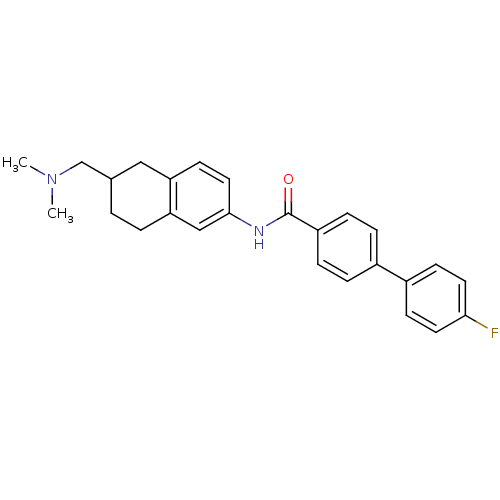 (4''-Fluoro-biphenyl-4-carboxylic acid (6-dimethyla...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302557 (CHEMBL565551 | N-((cis)-4-(4-(dimethylamino)-5-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302548 (3-chloro-N-((cis)-4-(4-(dimethylamino)-6-methylpyr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50170191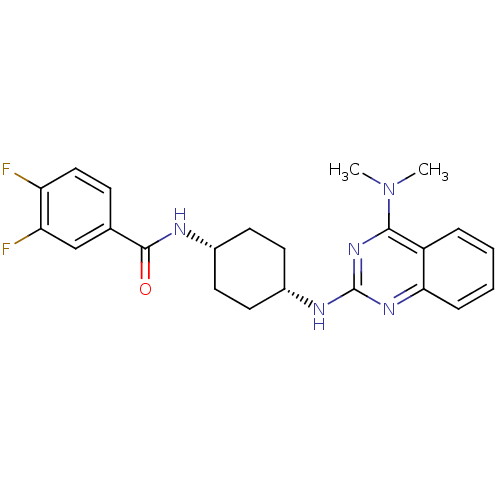 (CHEMBL182150 | N-((cis)-4-(4-(dimethylamino)quinaz...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302551 (CHEMBL567950 | N-((cis)-4-(4-(dimethylamino)-6-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302554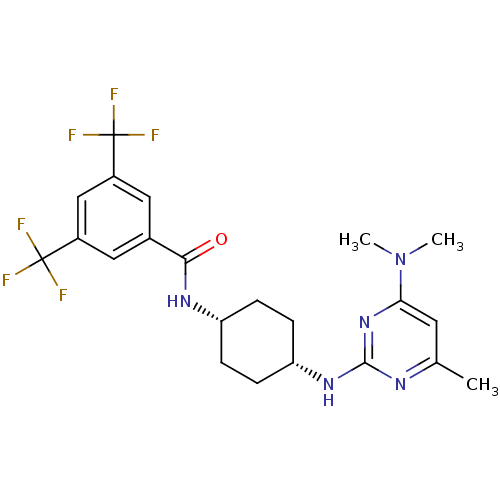 (CHEMBL583014 | N-((cis)-4-(4-(dimethylamino)-6-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302546 (CHEMBL568162 | N-((cis)-4-(4-(dimethylamino)-6-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50170191 (CHEMBL182150 | N-((cis)-4-(4-(dimethylamino)quinaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302558 (4-chloro-N-((cis)-4-(4-(dimethylamino)-5-methylpyr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235375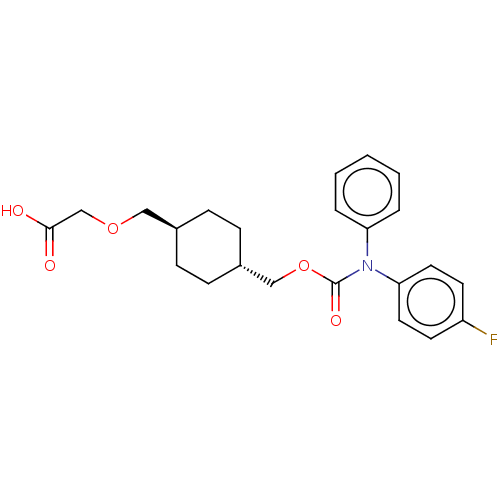 (CHEMBL3975122) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302556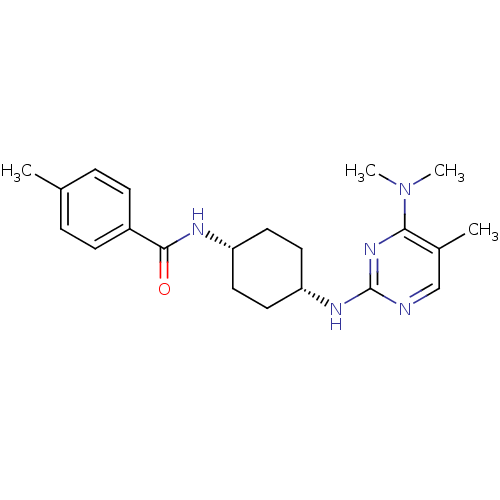 (CHEMBL578170 | N-((cis)-4-(4-(dimethylamino)-5-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302547 (4-chloro-N-((cis)-4-(4-(dimethylamino)-6-methylpyr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50170191 (CHEMBL182150 | N-((cis)-4-(4-(dimethylamino)quinaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 5HT1A | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235370 (CHEMBL3933704) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235378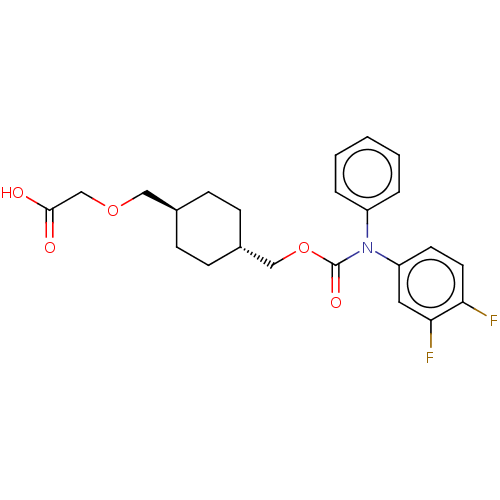 (CHEMBL3981509) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Major prion protein (Homo sapiens (Human)) | BDBM357271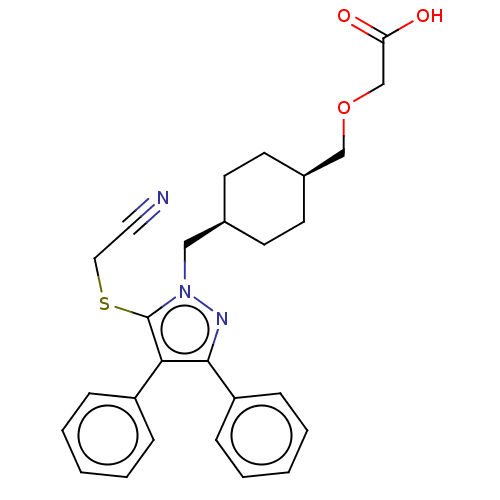 (2-(((1s,4s)-4-((5- (cyanomethylthio)-3,4- diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Blood collected from healthy human volunteers in aqueous trisodium citrate solution was centrifuged at 150 g for 15 min and the upper layer was recov... | US Patent US10214518 (2019) BindingDB Entry DOI: 10.7270/Q2T155XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235368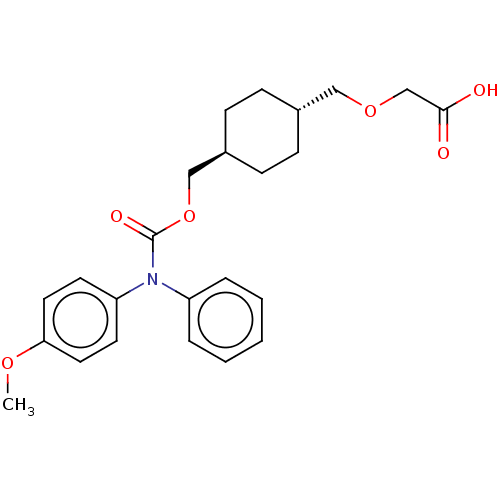 (CHEMBL3893346) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 358 total ) | Next | Last >> |