 Found 110 hits with Last Name = 'zych' and Initial = 'a'
Found 110 hits with Last Name = 'zych' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50065428
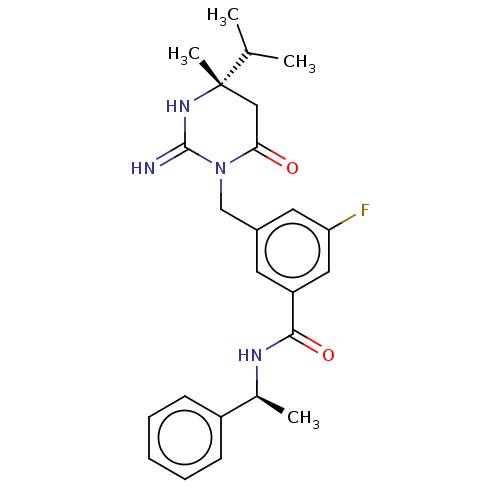
(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065395

(CHEMBL3401345)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2CC(CC2=O)c2cccc(Cl)c2)C(=N)N1 |r| Show InChI InChI=1S/C25H29ClN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-6-4-9-21(10-17)29-15-19(12-22(29)31)18-7-5-8-20(26)11-18/h4-11,16,19H,12-15H2,1-3H3,(H2,27,28)/t19?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065426
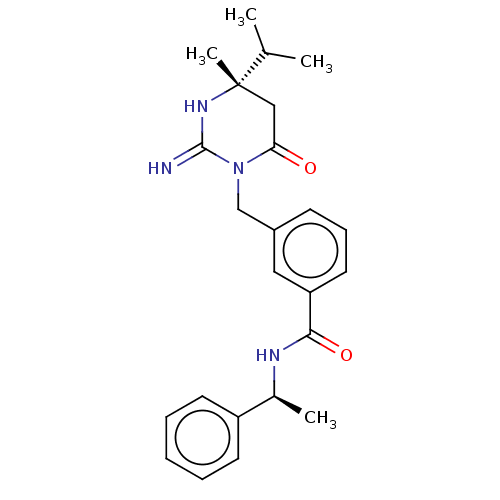
(CHEMBL3401348)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065424
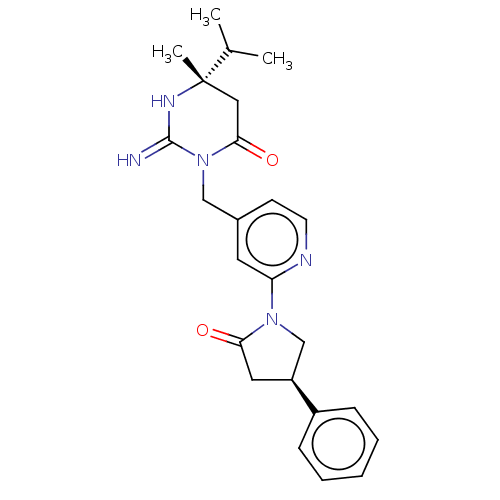
(CHEMBL3401346)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29N5O2/c1-16(2)24(3)13-22(31)29(23(25)27-24)14-17-9-10-26-20(11-17)28-15-19(12-21(28)30)18-7-5-4-6-8-18/h4-11,16,19H,12-15H2,1-3H3,(H2,25,27)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065425
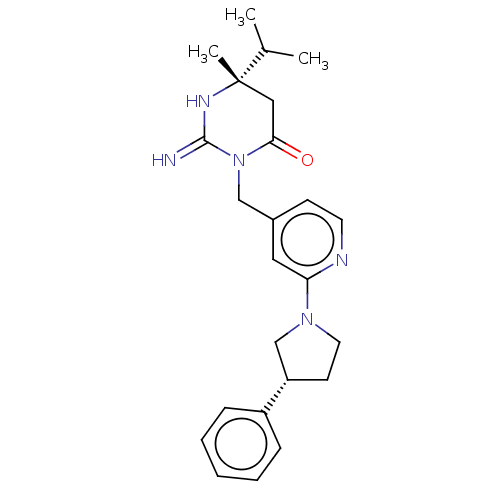
(CHEMBL3401347)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2CC[C@@H](C2)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H31N5O/c1-17(2)24(3)14-22(30)29(23(25)27-24)15-18-9-11-26-21(13-18)28-12-10-20(16-28)19-7-5-4-6-8-19/h4-9,11,13,17,20H,10,12,14-16H2,1-3H3,(H2,25,27)/t20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065393

(CHEMBL3401344)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H29FN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-9-20(26)12-21(10-17)29-15-19(11-22(29)31)18-7-5-4-6-8-18/h4-10,12,16,19H,11,13-15H2,1-3H3,(H2,27,28)/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065391
(CHEMBL3401342)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H30N4O2/c1-17(2)25(3)14-23(31)29(24(26)27-25)15-18-8-7-11-21(12-18)28-16-20(13-22(28)30)19-9-5-4-6-10-19/h4-12,17,20H,13-16H2,1-3H3,(H2,26,27)/t20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065427

(CHEMBL3401349)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065392
(CHEMBL3401343)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2C[C@@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H30N4O2/c1-17(2)25(3)14-23(31)29(24(26)27-25)15-18-8-7-11-21(12-18)28-16-20(13-22(28)30)19-9-5-4-6-10-19/h4-12,17,20H,13-16H2,1-3H3,(H2,26,27)/t20-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathE (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM98678
(US8497286, 154)Show SMILES CCOC[C@@H](CC(C)C)NC(=O)[C@@H]1CNC[C@@H]([C@@H]1O)C(=O)N(C1CC1)c1ccc(cn1)C(C)C |r| Show InChI InChI=1S/C26H42N4O4/c1-6-34-15-19(11-16(2)3)29-25(32)21-13-27-14-22(24(21)31)26(33)30(20-8-9-20)23-10-7-18(12-28-23)17(4)5/h7,10,12,16-17,19-22,24,27,31H,6,8-9,11,13-15H2,1-5H3,(H,29,32)/t19-,21-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50382334
(CHEMBL1276678)Show SMILES CN[C@H](CNC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)C[C@H]1CCCOC1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-23(14-19-6-5-12-35-18-19)16-30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50392953
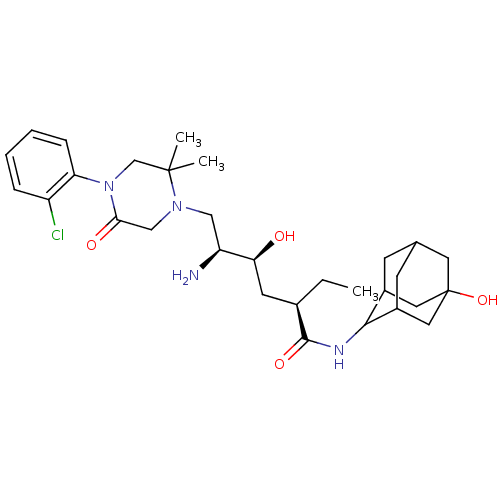
(CHEMBL2152353)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NC1C2CC3CC1CC(O)(C3)C2 |r,wU:4.4,2.2,wD:6.6,TLB:38:35:28.29.30:32,36:35:28:30.31.32,27:28:34.38.35:30.31.32,27:28:32:34.35.37,THB:38:29:32:34.35.37,37:35:28:30.31.32,37:31:28:34.38.35,(13.51,-6.54,;12.17,-7.31,;12.17,-8.85,;10.84,-9.62,;9.51,-8.85,;9.51,-7.31,;8.17,-9.62,;6.84,-8.85,;8.17,-11.16,;6.84,-11.93,;5.5,-11.17,;4.16,-11.94,;2.83,-11.17,;4.18,-13.47,;5.51,-14.24,;6.84,-13.48,;7.61,-14.81,;8.38,-13.48,;2.85,-14.25,;1.51,-13.49,;.18,-14.26,;.18,-15.8,;1.52,-16.57,;2.85,-15.8,;4.19,-16.57,;13.51,-9.62,;13.51,-11.16,;14.84,-8.86,;16.17,-9.63,;17.67,-9.21,;17.67,-7.62,;18.71,-6.39,;17.36,-6.87,;17.37,-8.35,;18.7,-8.84,;20.09,-8.5,;21.42,-9.26,;20.1,-6.97,;19.08,-9.77,)| Show InChI InChI=1S/C30H45ClN4O4/c1-4-19(28(38)33-27-20-9-18-10-21(27)14-30(39,12-18)13-20)11-25(36)23(32)15-34-16-26(37)35(17-29(34,2)3)24-8-6-5-7-22(24)31/h5-8,18-21,23,25,27,36,39H,4,9-17,32H2,1-3H3,(H,33,38)/t18?,19-,20?,21?,23+,25+,27?,30?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50347010
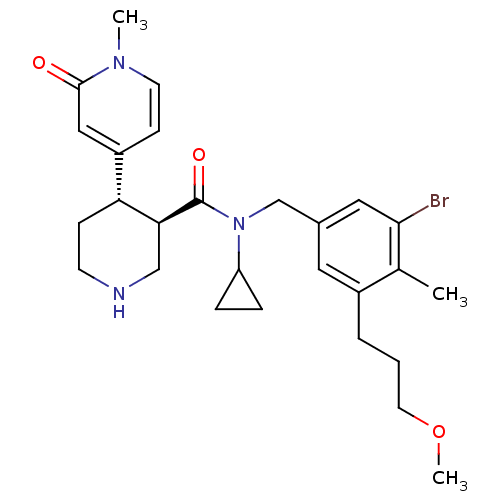
(CHEMBL1796063)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccn(C)c(=O)c2)cc(Br)c1C |r| Show InChI InChI=1S/C27H36BrN3O3/c1-18-20(5-4-12-34-3)13-19(14-25(18)28)17-31(22-6-7-22)27(33)24-16-29-10-8-23(24)21-9-11-30(2)26(32)15-21/h9,11,13-15,22-24,29H,4-8,10,12,16-17H2,1-3H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485379
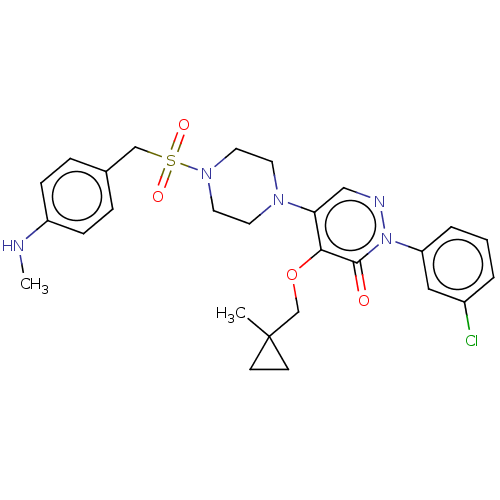
(CHEMBL2059641)Show SMILES CNc1ccc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cccc(Cl)c3)c(=O)c2OCC2(C)CC2)cc1 Show InChI InChI=1S/C27H32ClN5O4S/c1-27(10-11-27)19-37-25-24(17-30-33(26(25)34)23-5-3-4-21(28)16-23)31-12-14-32(15-13-31)38(35,36)18-20-6-8-22(29-2)9-7-20/h3-9,16-17,29H,10-15,18-19H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485394
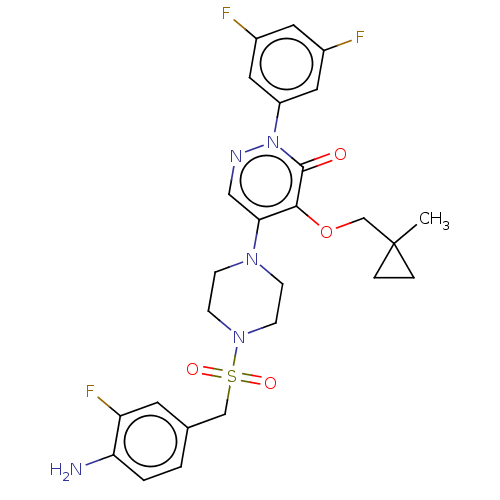
(CHEMBL2059493)Show SMILES CC1(COc2c(cnn(-c3cc(F)cc(F)c3)c2=O)N2CCN(CC2)S(=O)(=O)Cc2ccc(N)c(F)c2)CC1 Show InChI InChI=1S/C26H28F3N5O4S/c1-26(4-5-26)16-38-24-23(14-31-34(25(24)35)20-12-18(27)11-19(28)13-20)32-6-8-33(9-7-32)39(36,37)15-17-2-3-22(30)21(29)10-17/h2-3,10-14H,4-9,15-16,30H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485364
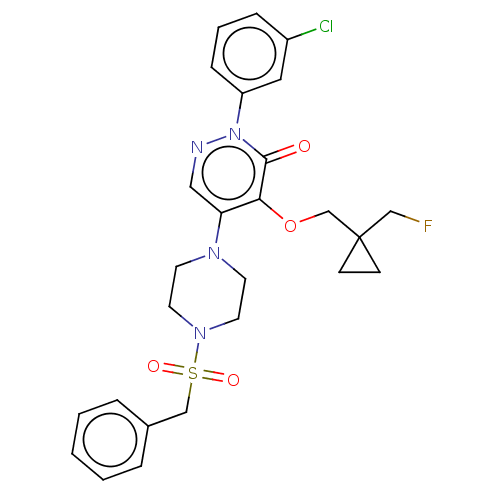
(CHEMBL2059474)Show SMILES FCC1(COc2c(cnn(-c3cccc(Cl)c3)c2=O)N2CCN(CC2)S(=O)(=O)Cc2ccccc2)CC1 Show InChI InChI=1S/C26H28ClFN4O4S/c27-21-7-4-8-22(15-21)32-25(33)24(36-19-26(18-28)9-10-26)23(16-29-32)30-11-13-31(14-12-30)37(34,35)17-20-5-2-1-3-6-20/h1-8,15-16H,9-14,17-19H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485398
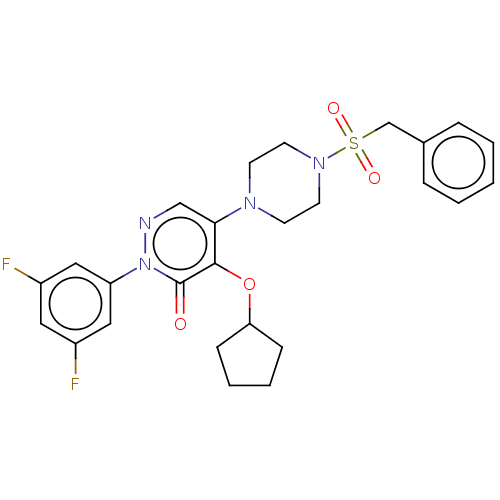
(CHEMBL2059384)Show SMILES Fc1cc(F)cc(c1)-n1ncc(N2CCN(CC2)S(=O)(=O)Cc2ccccc2)c(OC2CCCC2)c1=O Show InChI InChI=1S/C26H28F2N4O4S/c27-20-14-21(28)16-22(15-20)32-26(33)25(36-23-8-4-5-9-23)24(17-29-32)30-10-12-31(13-11-30)37(34,35)18-19-6-2-1-3-7-19/h1-3,6-7,14-17,23H,4-5,8-13,18H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485363
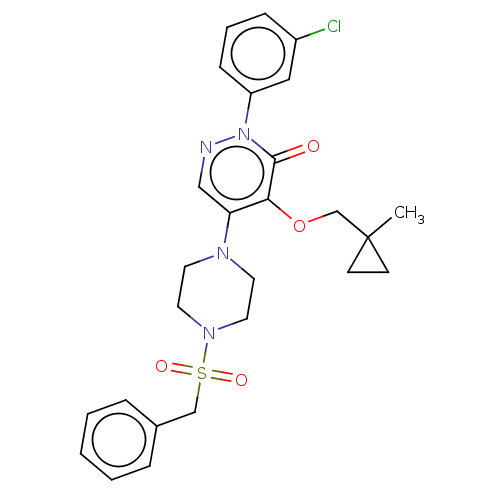
(CHEMBL2059473)Show SMILES CC1(COc2c(cnn(-c3cccc(Cl)c3)c2=O)N2CCN(CC2)S(=O)(=O)Cc2ccccc2)CC1 Show InChI InChI=1S/C26H29ClN4O4S/c1-26(10-11-26)19-35-24-23(17-28-31(25(24)32)22-9-5-8-21(27)16-22)29-12-14-30(15-13-29)36(33,34)18-20-6-3-2-4-7-20/h2-9,16-17H,10-15,18-19H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485376
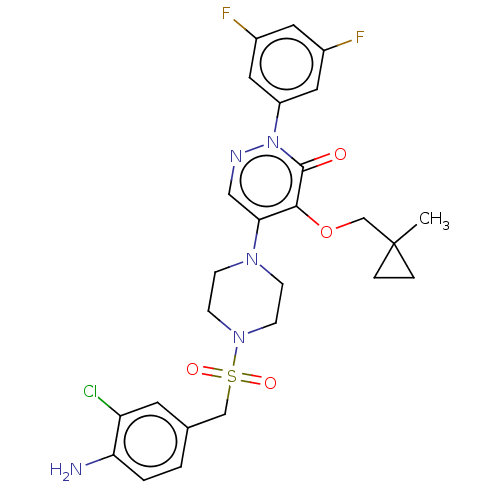
(CHEMBL2059494)Show SMILES CC1(COc2c(cnn(-c3cc(F)cc(F)c3)c2=O)N2CCN(CC2)S(=O)(=O)Cc2ccc(N)c(Cl)c2)CC1 Show InChI InChI=1S/C26H28ClF2N5O4S/c1-26(4-5-26)16-38-24-23(14-31-34(25(24)35)20-12-18(28)11-19(29)13-20)32-6-8-33(9-7-32)39(36,37)15-17-2-3-22(30)21(27)10-17/h2-3,10-14H,4-9,15-16,30H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485368
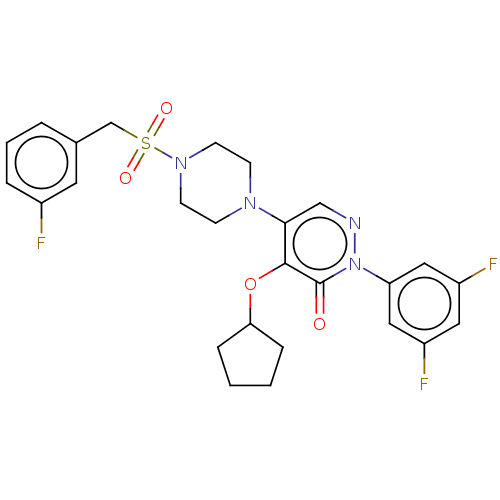
(CHEMBL2059481)Show SMILES Fc1cccc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cc(F)cc(F)c3)c(=O)c2OC2CCCC2)c1 Show InChI InChI=1S/C26H27F3N4O4S/c27-19-5-3-4-18(12-19)17-38(35,36)32-10-8-31(9-11-32)24-16-30-33(22-14-20(28)13-21(29)15-22)26(34)25(24)37-23-6-1-2-7-23/h3-5,12-16,23H,1-2,6-11,17H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485390
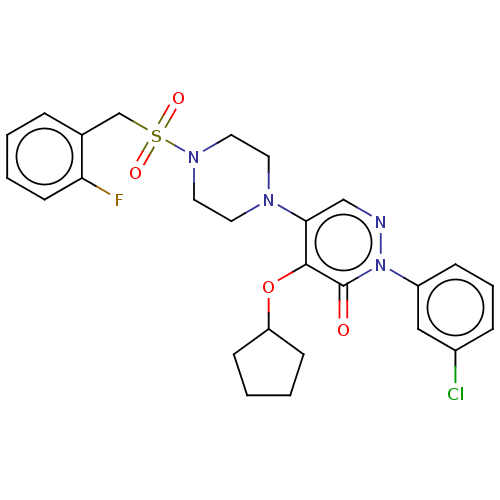
(CHEMBL2059482)Show SMILES Fc1ccccc1CS(=O)(=O)N1CCN(CC1)c1cnn(-c2cccc(Cl)c2)c(=O)c1OC1CCCC1 Show InChI InChI=1S/C26H28ClFN4O4S/c27-20-7-5-8-21(16-20)32-26(33)25(36-22-9-2-3-10-22)24(17-29-32)30-12-14-31(15-13-30)37(34,35)18-19-6-1-4-11-23(19)28/h1,4-8,11,16-17,22H,2-3,9-10,12-15,18H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485375
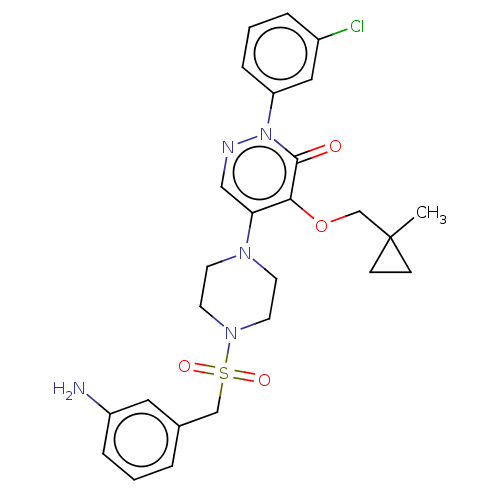
(CHEMBL2059492)Show SMILES CC1(COc2c(cnn(-c3cccc(Cl)c3)c2=O)N2CCN(CC2)S(=O)(=O)Cc2cccc(N)c2)CC1 Show InChI InChI=1S/C26H30ClN5O4S/c1-26(8-9-26)18-36-24-23(16-29-32(25(24)33)22-7-3-5-20(27)15-22)30-10-12-31(13-11-30)37(34,35)17-19-4-2-6-21(28)14-19/h2-7,14-16H,8-13,17-18,28H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485380
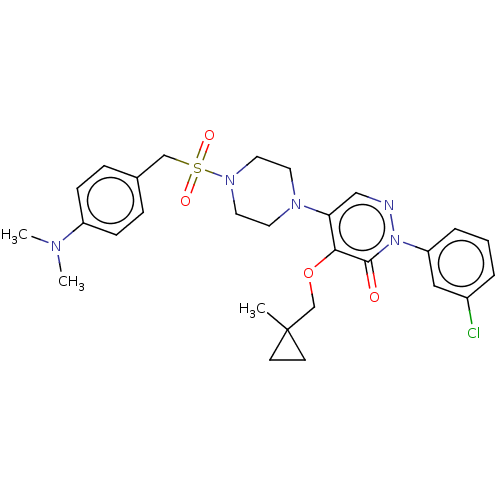
(CHEMBL2059642)Show SMILES CN(C)c1ccc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cccc(Cl)c3)c(=O)c2OCC2(C)CC2)cc1 Show InChI InChI=1S/C28H34ClN5O4S/c1-28(11-12-28)20-38-26-25(18-30-34(27(26)35)24-6-4-5-22(29)17-24)32-13-15-33(16-14-32)39(36,37)19-21-7-9-23(10-8-21)31(2)3/h4-10,17-18H,11-16,19-20H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485331
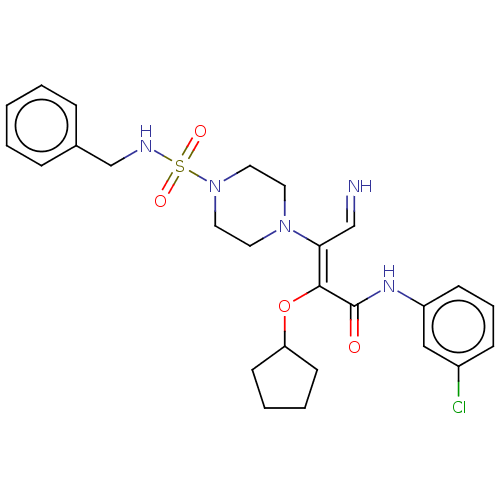
(CHEMBL2046603)Show SMILES Clc1cccc(NC(=O)C(\OC2CCCC2)=C(/C=N)N2CCN(CC2)S(=O)(=O)NCc2ccccc2)c1 Show InChI InChI=1S/C26H32ClN5O4S/c27-21-9-6-10-22(17-21)30-26(33)25(36-23-11-4-5-12-23)24(18-28)31-13-15-32(16-14-31)37(34,35)29-19-20-7-2-1-3-8-20/h1-3,6-10,17-18,23,28-29H,4-5,11-16,19H2,(H,30,33)/b25-24-,28-18? | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. (AMRI)
Curated by ChEMBL
| Assay Description
Inhibition of glucan synthase in Candida albicans BWP17 membrane |
Bioorg Med Chem Lett 22: 4896-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.127
BindingDB Entry DOI: 10.7270/Q2NC6424 |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485333
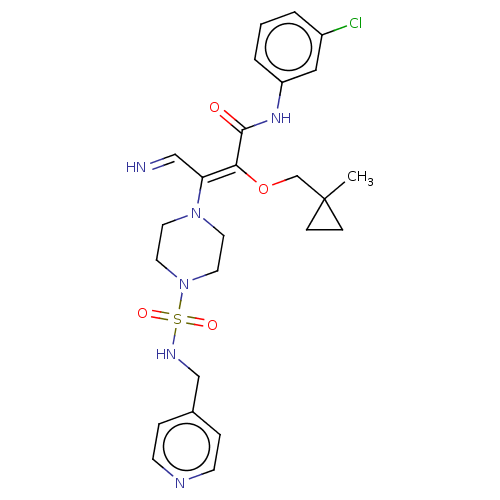
(CHEMBL2046738)Show SMILES CC1(CO\C(C(=O)Nc2cccc(Cl)c2)=C(\C=N)N2CCN(CC2)S(=O)(=O)NCc2ccncc2)CC1 Show InChI InChI=1S/C25H31ClN6O4S/c1-25(7-8-25)18-36-23(24(33)30-21-4-2-3-20(26)15-21)22(16-27)31-11-13-32(14-12-31)37(34,35)29-17-19-5-9-28-10-6-19/h2-6,9-10,15-16,27,29H,7-8,11-14,17-18H2,1H3,(H,30,33)/b23-22-,27-16? | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. (AMRI)
Curated by ChEMBL
| Assay Description
Inhibition of glucan synthase in Candida albicans BWP17 membrane |
Bioorg Med Chem Lett 22: 4896-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.127
BindingDB Entry DOI: 10.7270/Q2NC6424 |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485393
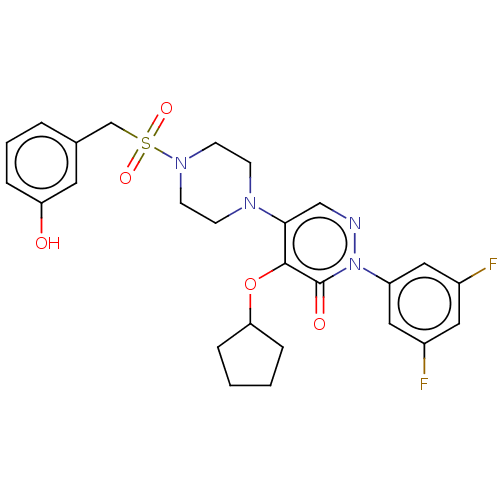
(CHEMBL2059490)Show SMILES Oc1cccc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cc(F)cc(F)c3)c(=O)c2OC2CCCC2)c1 Show InChI InChI=1S/C26H28F2N4O5S/c27-19-13-20(28)15-21(14-19)32-26(34)25(37-23-6-1-2-7-23)24(16-29-32)30-8-10-31(11-9-30)38(35,36)17-18-4-3-5-22(33)12-18/h3-5,12-16,23,33H,1-2,6-11,17H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485396
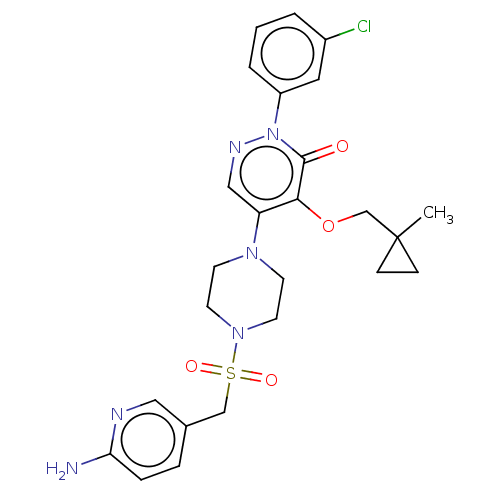
(CHEMBL2059643)Show SMILES CC1(COc2c(cnn(-c3cccc(Cl)c3)c2=O)N2CCN(CC2)S(=O)(=O)Cc2ccc(N)nc2)CC1 Show InChI InChI=1S/C25H29ClN6O4S/c1-25(7-8-25)17-36-23-21(15-29-32(24(23)33)20-4-2-3-19(26)13-20)30-9-11-31(12-10-30)37(34,35)16-18-5-6-22(27)28-14-18/h2-6,13-15H,7-12,16-17H2,1H3,(H2,27,28) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485392
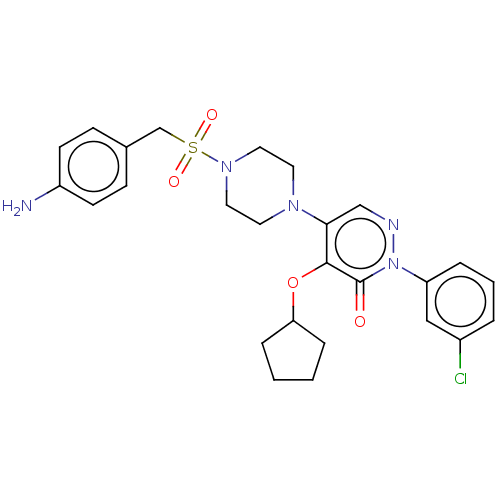
(CHEMBL1770665)Show SMILES Nc1ccc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cccc(Cl)c3)c(=O)c2OC2CCCC2)cc1 Show InChI InChI=1S/C26H30ClN5O4S/c27-20-4-3-5-22(16-20)32-26(33)25(36-23-6-1-2-7-23)24(17-29-32)30-12-14-31(15-13-30)37(34,35)18-19-8-10-21(28)11-9-19/h3-5,8-11,16-17,23H,1-2,6-7,12-15,18,28H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485327

(CHEMBL2046736)Show SMILES Cc1nc2ccc(CNS(=O)(=O)N3CCN(CC3)C(\C=N)=C(/OCC3(C)CC3)C(=O)Nc3cccc(Cl)c3)cc2s1 Show InChI InChI=1S/C28H33ClN6O4S2/c1-19-32-23-7-6-20(14-25(23)40-19)17-31-41(37,38)35-12-10-34(11-13-35)24(16-30)26(39-18-28(2)8-9-28)27(36)33-22-5-3-4-21(29)15-22/h3-7,14-16,30-31H,8-13,17-18H2,1-2H3,(H,33,36)/b26-24-,30-16? | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. (AMRI)
Curated by ChEMBL
| Assay Description
Inhibition of glucan synthase in Candida albicans BWP17 membrane |
Bioorg Med Chem Lett 22: 4896-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.127
BindingDB Entry DOI: 10.7270/Q2NC6424 |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485400
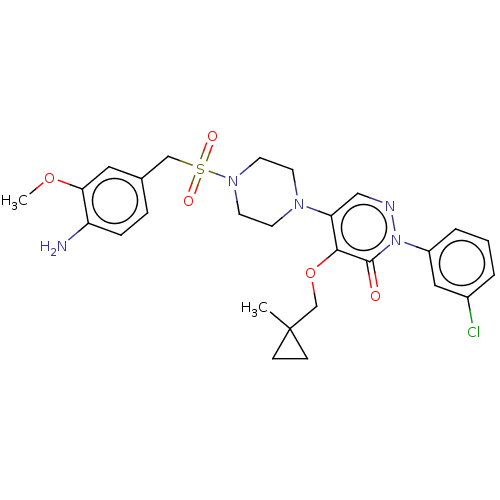
(CHEMBL2059496)Show SMILES COc1cc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cccc(Cl)c3)c(=O)c2OCC2(C)CC2)ccc1N Show InChI InChI=1S/C27H32ClN5O5S/c1-27(8-9-27)18-38-25-23(16-30-33(26(25)34)21-5-3-4-20(28)15-21)31-10-12-32(13-11-31)39(35,36)17-19-6-7-22(29)24(14-19)37-2/h3-7,14-16H,8-13,17-18,29H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485326
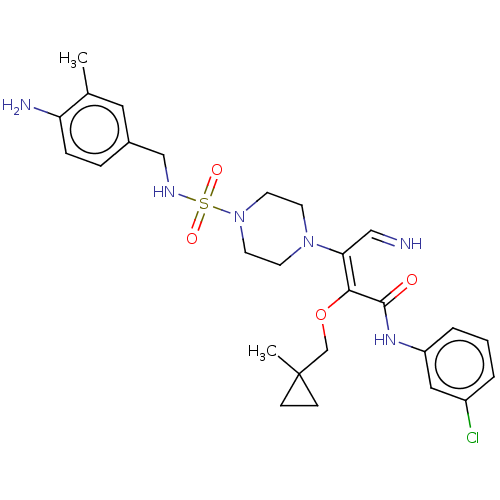
(CHEMBL2046747)Show SMILES Cc1cc(CNS(=O)(=O)N2CCN(CC2)C(\C=N)=C(/OCC2(C)CC2)C(=O)Nc2cccc(Cl)c2)ccc1N Show InChI InChI=1S/C27H35ClN6O4S/c1-19-14-20(6-7-23(19)30)17-31-39(36,37)34-12-10-33(11-13-34)24(16-29)25(38-18-27(2)8-9-27)26(35)32-22-5-3-4-21(28)15-22/h3-7,14-16,29,31H,8-13,17-18,30H2,1-2H3,(H,32,35)/b25-24-,29-16? | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. (AMRI)
Curated by ChEMBL
| Assay Description
Inhibition of glucan synthase in Candida albicans BWP17 membrane |
Bioorg Med Chem Lett 22: 4896-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.127
BindingDB Entry DOI: 10.7270/Q2NC6424 |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485367

(CHEMBL1770516)Show SMILES Fc1ccc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cccc(Cl)c3)c(=O)c2OC2CCCC2)cc1 Show InChI InChI=1S/C26H28ClFN4O4S/c27-20-4-3-5-22(16-20)32-26(33)25(36-23-6-1-2-7-23)24(17-29-32)30-12-14-31(15-13-30)37(34,35)18-19-8-10-21(28)11-9-19/h3-5,8-11,16-17,23H,1-2,6-7,12-15,18H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485372
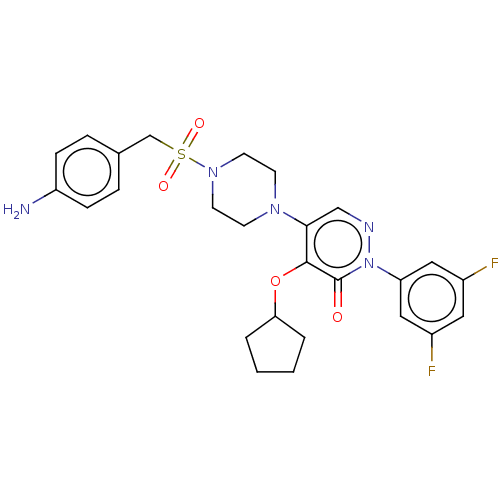
(CHEMBL2059488)Show SMILES Nc1ccc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cc(F)cc(F)c3)c(=O)c2OC2CCCC2)cc1 Show InChI InChI=1S/C26H29F2N5O4S/c27-19-13-20(28)15-22(14-19)33-26(34)25(37-23-3-1-2-4-23)24(16-30-33)31-9-11-32(12-10-31)38(35,36)17-18-5-7-21(29)8-6-18/h5-8,13-16,23H,1-4,9-12,17,29H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485336
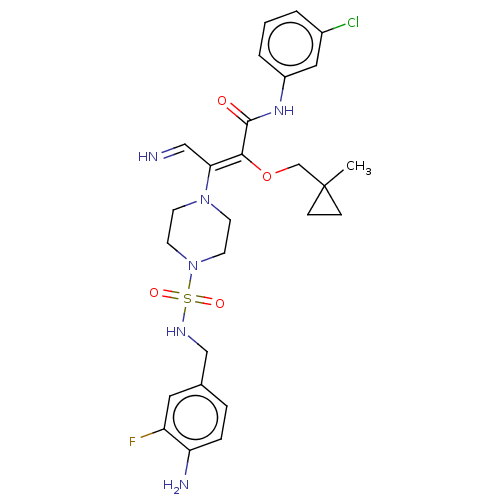
(CHEMBL2046746)Show SMILES CC1(CO\C(C(=O)Nc2cccc(Cl)c2)=C(\C=N)N2CCN(CC2)S(=O)(=O)NCc2ccc(N)c(F)c2)CC1 Show InChI InChI=1S/C26H32ClFN6O4S/c1-26(7-8-26)17-38-24(25(35)32-20-4-2-3-19(27)14-20)23(15-29)33-9-11-34(12-10-33)39(36,37)31-16-18-5-6-22(30)21(28)13-18/h2-6,13-15,29,31H,7-12,16-17,30H2,1H3,(H,32,35)/b24-23-,29-15? | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. (AMRI)
Curated by ChEMBL
| Assay Description
Inhibition of glucan synthase in Candida albicans BWP17 membrane |
Bioorg Med Chem Lett 22: 4896-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.127
BindingDB Entry DOI: 10.7270/Q2NC6424 |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485374
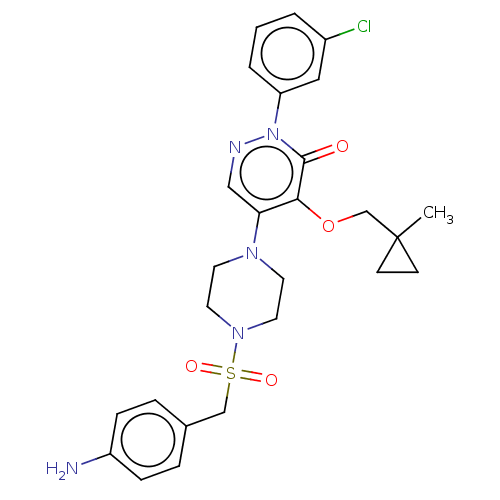
(CHEMBL2059491)Show SMILES CC1(COc2c(cnn(-c3cccc(Cl)c3)c2=O)N2CCN(CC2)S(=O)(=O)Cc2ccc(N)cc2)CC1 Show InChI InChI=1S/C26H30ClN5O4S/c1-26(9-10-26)18-36-24-23(16-29-32(25(24)33)22-4-2-3-20(27)15-22)30-11-13-31(14-12-30)37(34,35)17-19-5-7-21(28)8-6-19/h2-8,15-16H,9-14,17-18,28H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485328
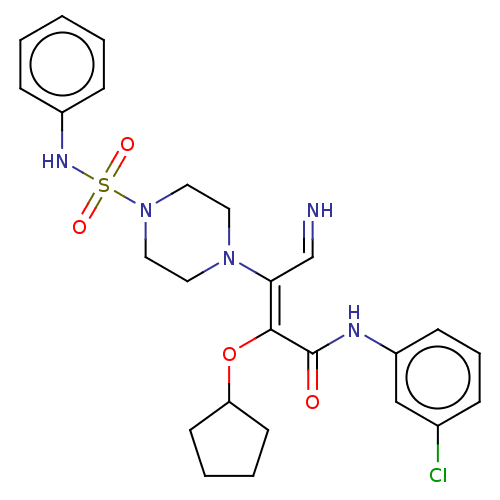
(CHEMBL2046602)Show SMILES Clc1cccc(NC(=O)C(\OC2CCCC2)=C(/C=N)N2CCN(CC2)S(=O)(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C25H30ClN5O4S/c26-19-7-6-10-21(17-19)28-25(32)24(35-22-11-4-5-12-22)23(18-27)30-13-15-31(16-14-30)36(33,34)29-20-8-2-1-3-9-20/h1-3,6-10,17-18,22,27,29H,4-5,11-16H2,(H,28,32)/b24-23-,27-18? | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. (AMRI)
Curated by ChEMBL
| Assay Description
Inhibition of glucan synthase in Candida albicans BWP17 membrane |
Bioorg Med Chem Lett 22: 4896-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.127
BindingDB Entry DOI: 10.7270/Q2NC6424 |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485338
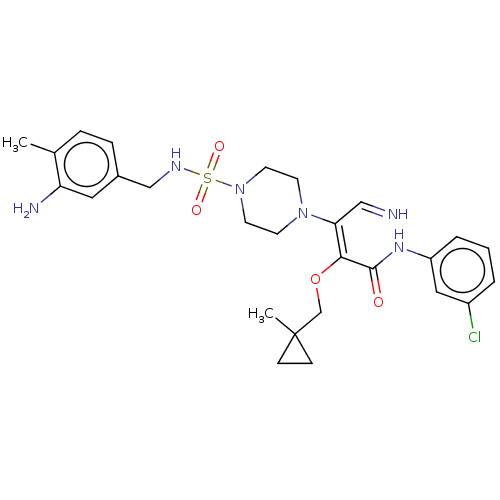
(CHEMBL2046742)Show SMILES Cc1ccc(CNS(=O)(=O)N2CCN(CC2)C(\C=N)=C(/OCC2(C)CC2)C(=O)Nc2cccc(Cl)c2)cc1N Show InChI InChI=1S/C27H35ClN6O4S/c1-19-6-7-20(14-23(19)30)17-31-39(36,37)34-12-10-33(11-13-34)24(16-29)25(38-18-27(2)8-9-27)26(35)32-22-5-3-4-21(28)15-22/h3-7,14-16,29,31H,8-13,17-18,30H2,1-2H3,(H,32,35)/b25-24-,29-16? | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. (AMRI)
Curated by ChEMBL
| Assay Description
Inhibition of glucan synthase in Candida albicans BWP17 membrane |
Bioorg Med Chem Lett 22: 4896-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.127
BindingDB Entry DOI: 10.7270/Q2NC6424 |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485397
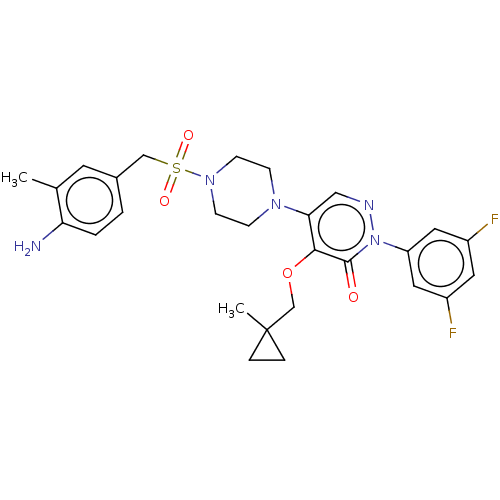
(CHEMBL2059637)Show SMILES Cc1cc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cc(F)cc(F)c3)c(=O)c2OCC2(C)CC2)ccc1N Show InChI InChI=1S/C27H31F2N5O4S/c1-18-11-19(3-4-23(18)30)16-39(36,37)33-9-7-32(8-10-33)24-15-31-34(22-13-20(28)12-21(29)14-22)26(35)25(24)38-17-27(2)5-6-27/h3-4,11-15H,5-10,16-17,30H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |
1,3-beta-D-glucan synthase catalytic subunit
(Candida albicans) | BDBM50485373
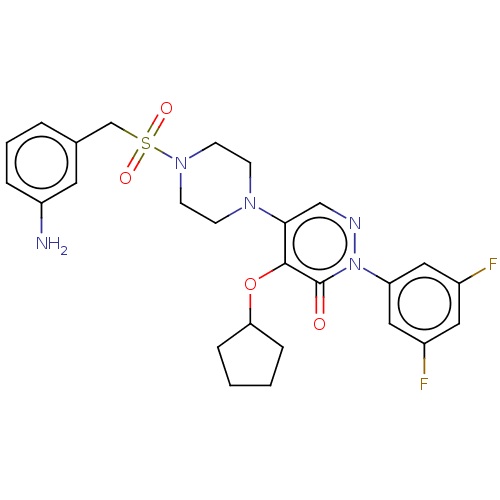
(CHEMBL2059489)Show SMILES Nc1cccc(CS(=O)(=O)N2CCN(CC2)c2cnn(-c3cc(F)cc(F)c3)c(=O)c2OC2CCCC2)c1 Show InChI InChI=1S/C26H29F2N5O4S/c27-19-13-20(28)15-22(14-19)33-26(34)25(37-23-6-1-2-7-23)24(16-30-33)31-8-10-32(11-9-31)38(35,36)17-18-4-3-5-21(29)12-18/h3-5,12-16,23H,1-2,6-11,17,29H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase in Candida albicans BWP17 membranes |
Bioorg Med Chem Lett 22: 5268-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.091
BindingDB Entry DOI: 10.7270/Q2MC92WW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data