

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50203827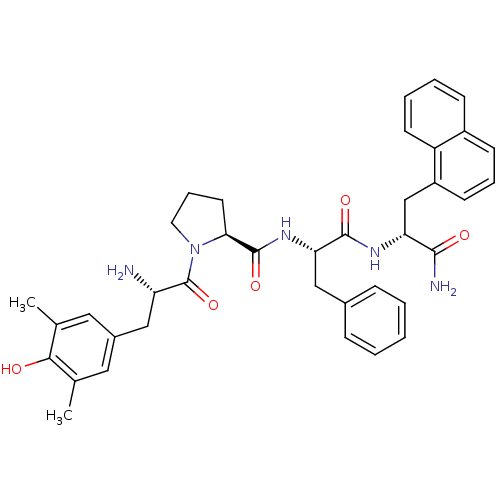 (CHEMBL385583 | Dmt-Pro-Phe-D-1-Nal-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane | J Med Chem 50: 512-20 (2007) Article DOI: 10.1021/jm060998u BindingDB Entry DOI: 10.7270/Q28915JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50232769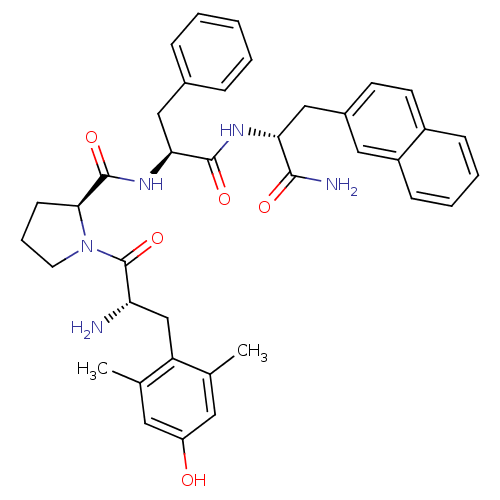 (CHEMBL254919 | Dmt-Pro-Phe-D-2-Nal-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane | J Med Chem 50: 512-20 (2007) Article DOI: 10.1021/jm060998u BindingDB Entry DOI: 10.7270/Q28915JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50203823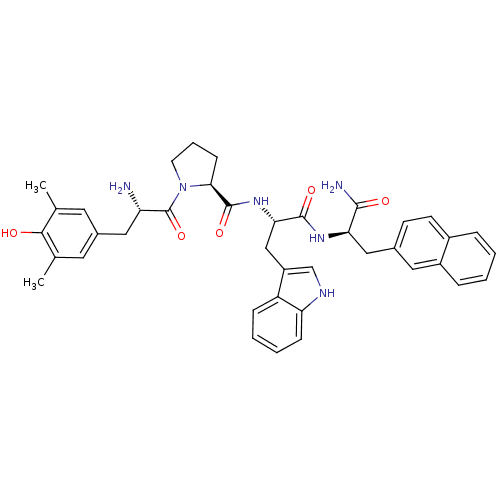 (CHEMBL218433 | Dmt-Pro-Trp-D-2-Nal-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane | J Med Chem 50: 512-20 (2007) Article DOI: 10.1021/jm060998u BindingDB Entry DOI: 10.7270/Q28915JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50232769 (CHEMBL254919 | Dmt-Pro-Phe-D-2-Nal-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane | J Med Chem 50: 512-20 (2007) Article DOI: 10.1021/jm060998u BindingDB Entry DOI: 10.7270/Q28915JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50203823 (CHEMBL218433 | Dmt-Pro-Trp-D-2-Nal-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane | J Med Chem 50: 512-20 (2007) Article DOI: 10.1021/jm060998u BindingDB Entry DOI: 10.7270/Q28915JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50203827 (CHEMBL385583 | Dmt-Pro-Phe-D-1-Nal-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane | J Med Chem 50: 512-20 (2007) Article DOI: 10.1021/jm060998u BindingDB Entry DOI: 10.7270/Q28915JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50229666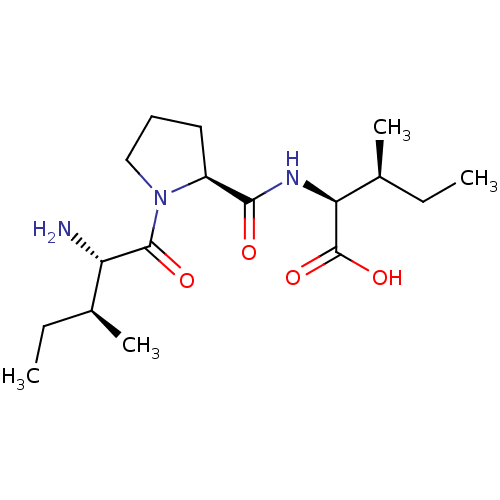 ((2S,3S)-2-((S)-1-((2S,3S)-2-amino-3-methylpentanoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DPP-4 (unknown origin) assessed as inhibition of endomorphin-2 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50229666 ((2S,3S)-2-((S)-1-((2S,3S)-2-amino-3-methylpentanoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DPP-4 (unknown origin) assessed as inhibition of endomorphin-1 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50487907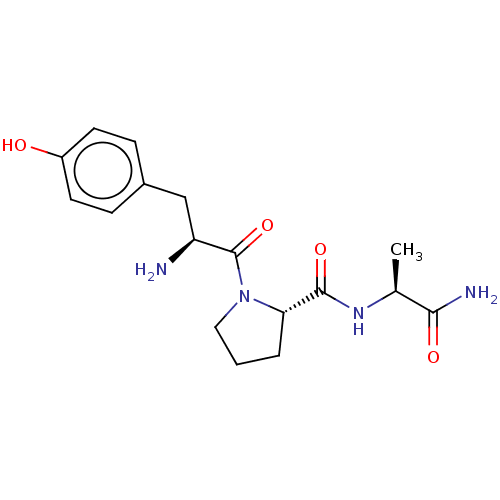 (CHEMBL2261078) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of APM (unknown origin) assessed as inhibition of endomorphin-2 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50487906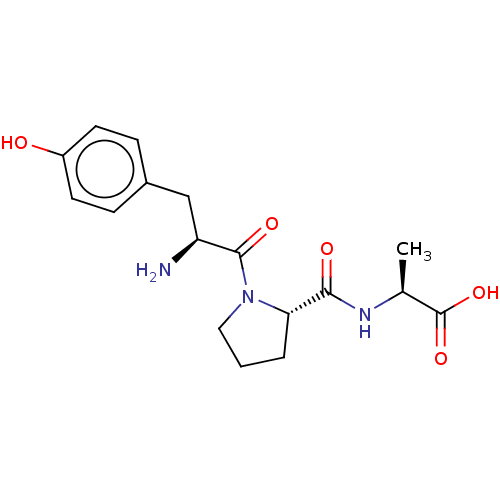 (CHEMBL2261077) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of APM (unknown origin) assessed as inhibition of endomorphin-1 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50487906 (CHEMBL2261077) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DPP-4 (unknown origin) assessed as inhibition of endomorphin-2 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50487907 (CHEMBL2261078) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of APM (unknown origin) assessed as inhibition of endomorphin-1 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50487907 (CHEMBL2261078) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DPP-4 (unknown origin) assessed as inhibition of endomorphin-2 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50487906 (CHEMBL2261077) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DPP-4 (unknown origin) assessed as inhibition of endomorphin-1 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50487906 (CHEMBL2261077) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of APM (unknown origin) assessed as inhibition of endomorphin-2 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50089194 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of APM (unknown origin) assessed as inhibition of endomorphin-2 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50089194 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of APM (unknown origin) assessed as inhibition of endomorphin-1 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50487907 (CHEMBL2261078) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DPP-4 (unknown origin) assessed as inhibition of endomorphin-1 degradation after 30 min | Med Chem Res 21: 1445-1450 (2012) Article DOI: 10.1007/s00044-011-9666-5 BindingDB Entry DOI: 10.7270/Q2NP27BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50232770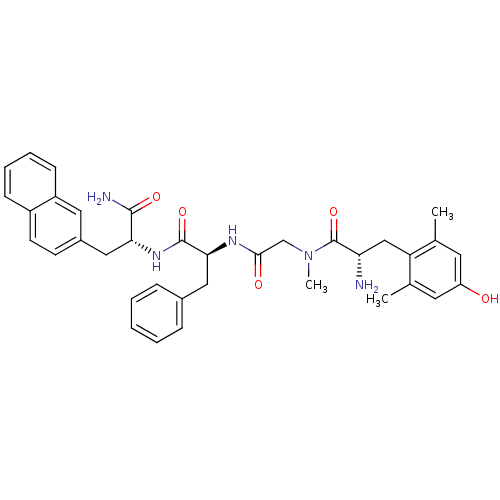 ((S)-2-Amino-N-{[(S)-1-((R)-1-carbamoyl-2-naphthale...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from mu opioid receptor in rat brain membranes | Bioorg Med Chem Lett 18: 1350-3 (2008) Article DOI: 10.1016/j.bmcl.2008.01.009 BindingDB Entry DOI: 10.7270/Q2QR4WWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50278897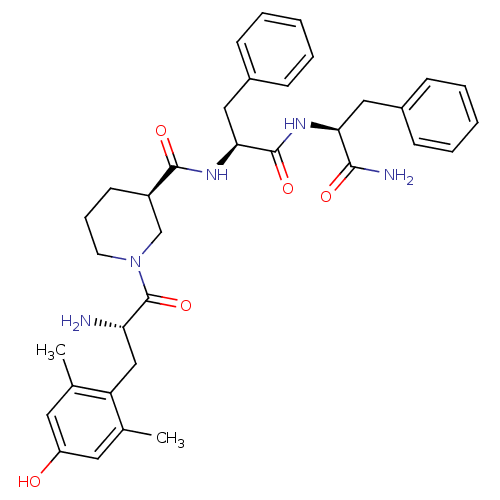 ((R)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membrane | Bioorg Med Chem 17: 3789-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.046 BindingDB Entry DOI: 10.7270/Q2BV7GHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50278895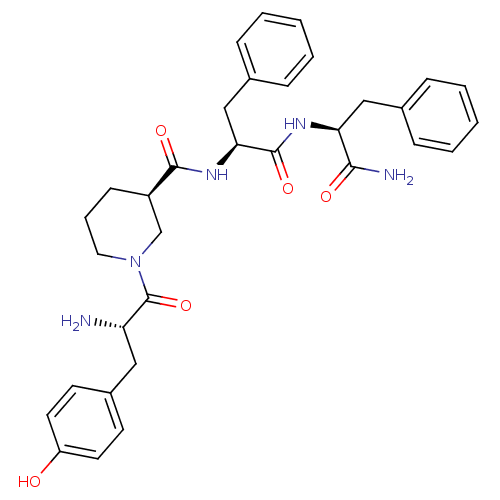 ((R)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membrane | Bioorg Med Chem 17: 3789-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.046 BindingDB Entry DOI: 10.7270/Q2BV7GHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50359547 (CHEMBL1927271) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from Wistar rat mu opioid receptor by liquid scintillation counting | Bioorg Med Chem 19: 6977-81 (2011) Article DOI: 10.1016/j.bmc.2011.10.040 BindingDB Entry DOI: 10.7270/Q2Q52Q1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50096787 (CHEMBL3580749) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from kappa opioid receptor in guinea pig brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50278893 ((R)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membrane | Bioorg Med Chem 17: 3789-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.046 BindingDB Entry DOI: 10.7270/Q2BV7GHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50092198 (CHEMBL3580743) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membrane | Bioorg Med Chem 17: 3789-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.046 BindingDB Entry DOI: 10.7270/Q2BV7GHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096787 (CHEMBL3580749) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096744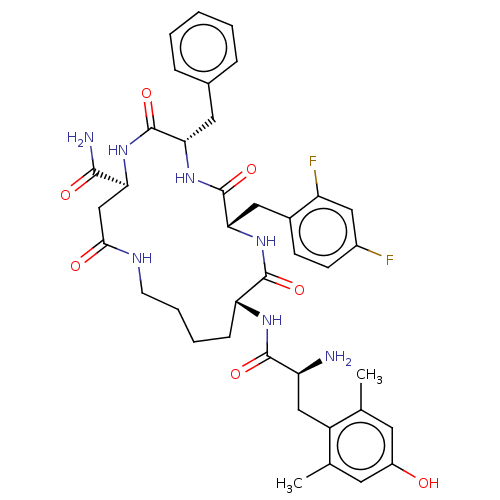 (CHEMBL3580746) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096745 (CHEMBL3580745) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50096736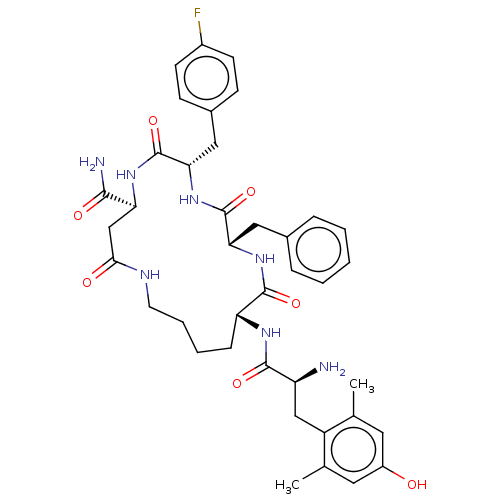 (CHEMBL3580748) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from kappa opioid receptor in guinea pig brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468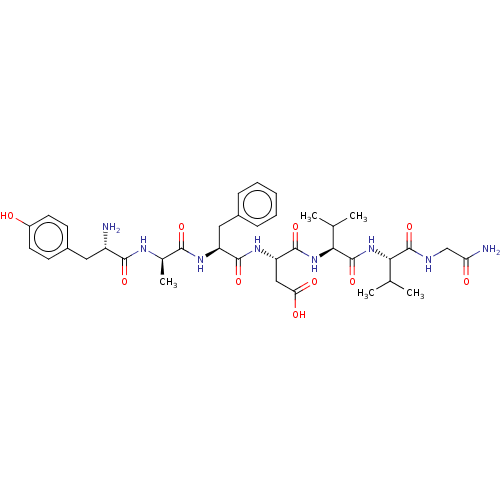 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 18: 1350-3 (2008) Article DOI: 10.1016/j.bmcl.2008.01.009 BindingDB Entry DOI: 10.7270/Q2QR4WWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50359546 (CHEMBL1927270) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from Wistar rat mu opioid receptor by liquid scintillation counting | Bioorg Med Chem 19: 6977-81 (2011) Article DOI: 10.1016/j.bmc.2011.10.040 BindingDB Entry DOI: 10.7270/Q2Q52Q1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50092199 (CHEMBL3582487) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from kappa opioid receptor in guinea pig brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50096744 (CHEMBL3580746) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from kappa opioid receptor in guinea pig brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096736 (CHEMBL3580748) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membrane | Bioorg Med Chem 17: 3789-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.046 BindingDB Entry DOI: 10.7270/Q2BV7GHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096789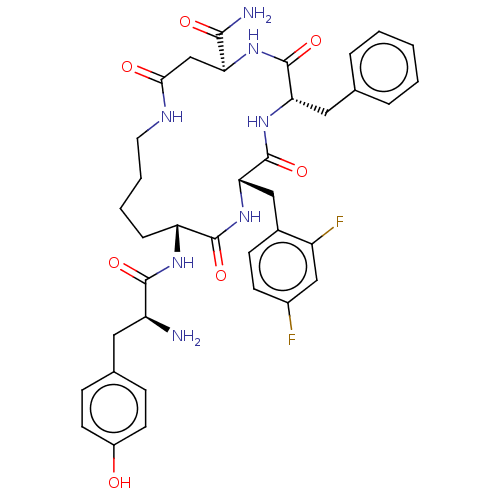 (CHEMBL3582485) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096788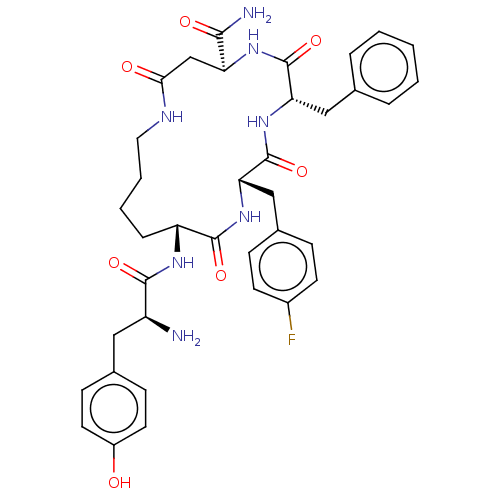 (CHEMBL3582484) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from mu opioid receptor in rat brain membranes | Bioorg Med Chem Lett 18: 1350-3 (2008) Article DOI: 10.1016/j.bmcl.2008.01.009 BindingDB Entry DOI: 10.7270/Q2QR4WWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50092198 (CHEMBL3580743) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from kappa opioid receptor in guinea pig brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096786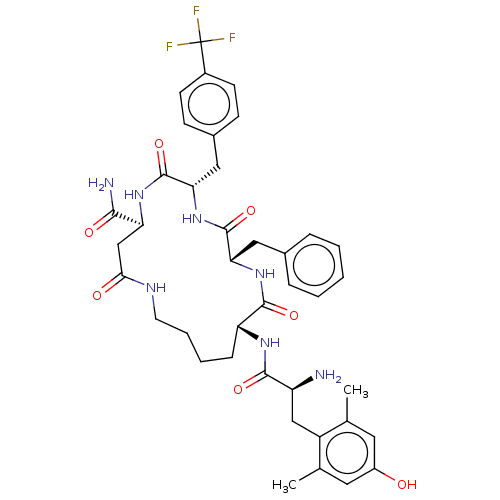 (CHEMBL3580750) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50092199 (CHEMBL3582487) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from Wistar rat mu opioid receptor by liquid scintillation counting | Bioorg Med Chem 19: 6977-81 (2011) Article DOI: 10.1016/j.bmc.2011.10.040 BindingDB Entry DOI: 10.7270/Q2Q52Q1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50096786 (CHEMBL3580750) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from kappa opioid receptor in guinea pig brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50347818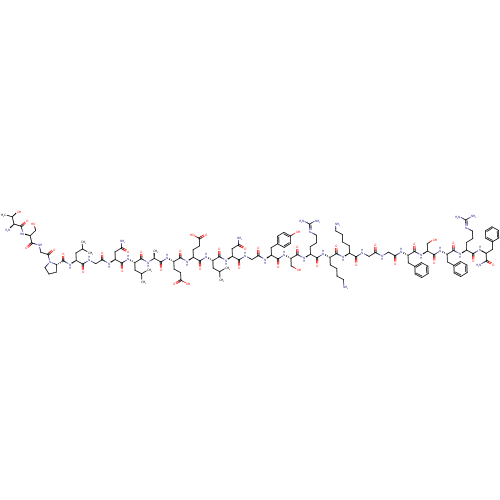 (CHEMBL1802413 | P518) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]26RFa from human GPR103 expressed in HEK293 cells incubated for 1 h by gamma counter based method | J Med Chem 55: 7516-24 (2012) Article DOI: 10.1021/jm300507d BindingDB Entry DOI: 10.7270/Q2K35VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50096791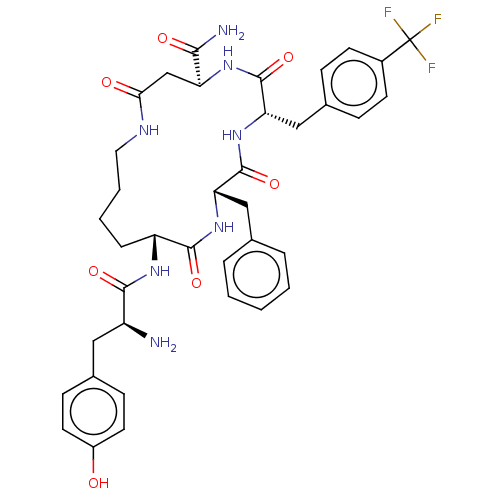 (CHEMBL3580744) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from kappa opioid receptor in guinea pig brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50232769 (CHEMBL254919 | Dmt-Pro-Phe-D-2-Nal-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from mu opioid receptor in rat brain membranes | Bioorg Med Chem Lett 18: 1350-3 (2008) Article DOI: 10.1016/j.bmcl.2008.01.009 BindingDB Entry DOI: 10.7270/Q2QR4WWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096791 (CHEMBL3580744) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50096788 (CHEMBL3582484) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from kappa opioid receptor in guinea pig brain membranes after 120 mins by scintillation counting analysis | ACS Med Chem Lett 6: 579-83 (2015) Article DOI: 10.1021/acsmedchemlett.5b00056 BindingDB Entry DOI: 10.7270/Q2FF3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |