

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 312.3 BDBM16658  Purchase Purchase | Wt: 326.3 BDBM16659 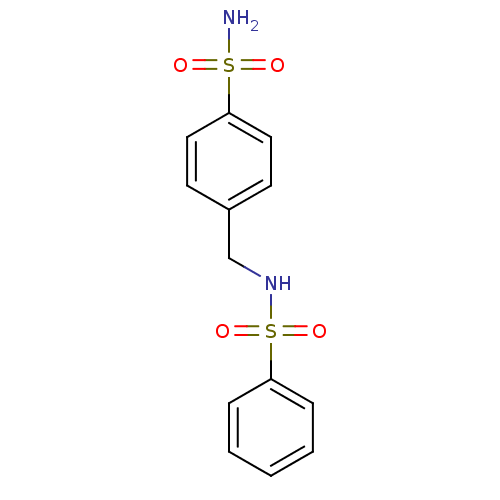 Purchase Purchase | Wt: 340.4 BDBM16660 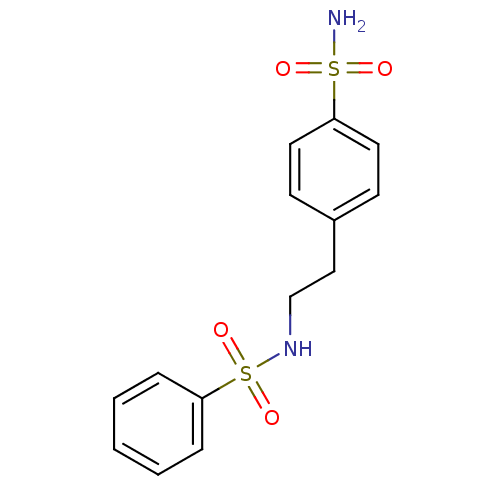 Purchase Purchase | Wt: 330.3 BDBM16661 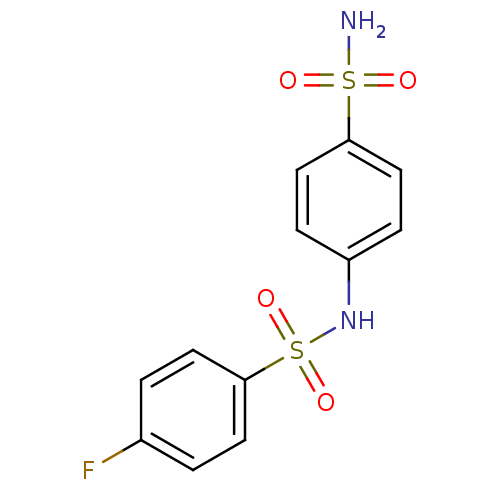 Purchase Purchase | Wt: 357.3 BDBM16662 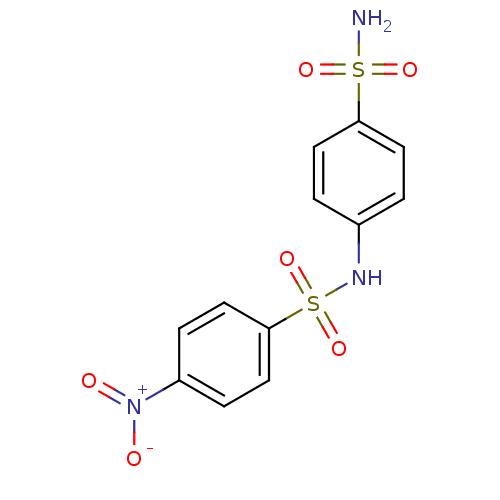 Purchase Purchase |
| Wt: 397.4 BDBM16663 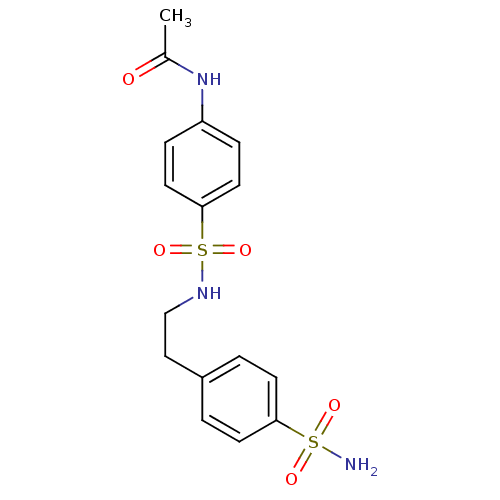 Purchase Purchase | Wt: 344.3 BDBM16664 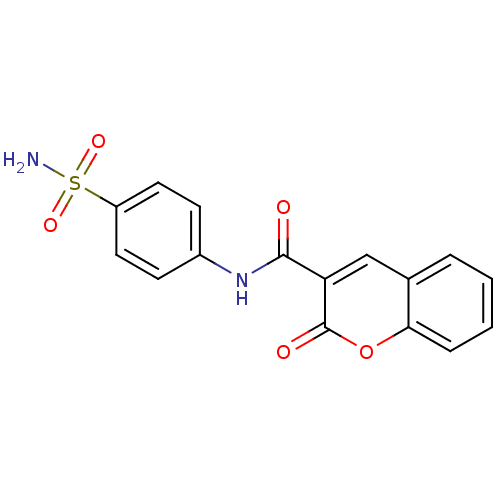 Purchase Purchase | Wt: 358.3 BDBM16665 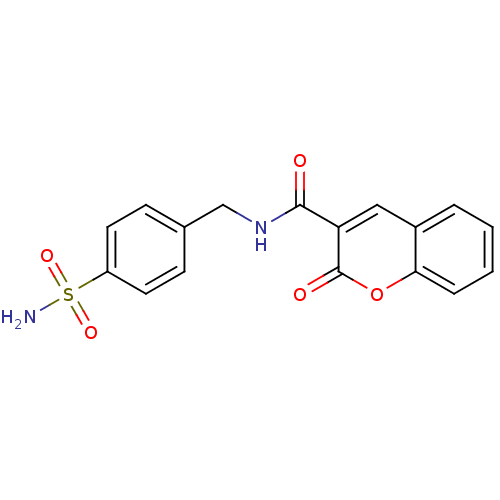 Purchase Purchase | Wt: 372.3 BDBM16666 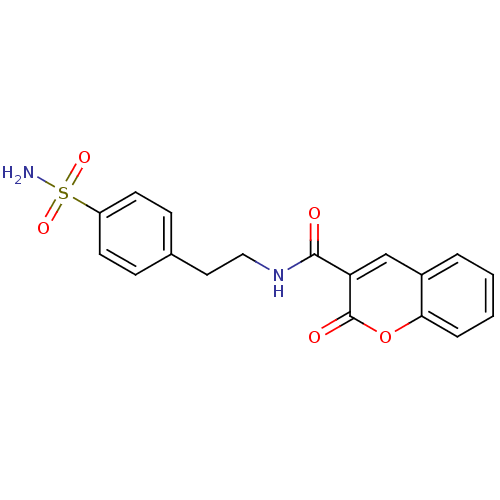 Purchase Purchase | Wt: 214.2 BDBM16643 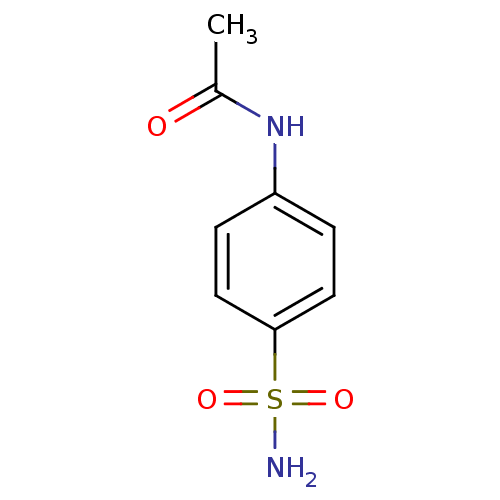 Purchase Purchase |
| Wt: 268.2 BDBM16644 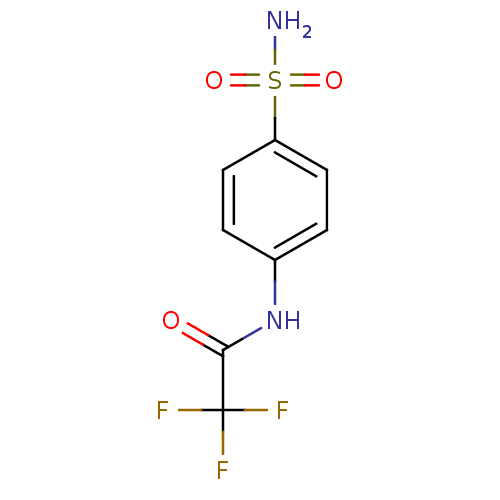 Purchase Purchase | Wt: 228.2 BDBM16645 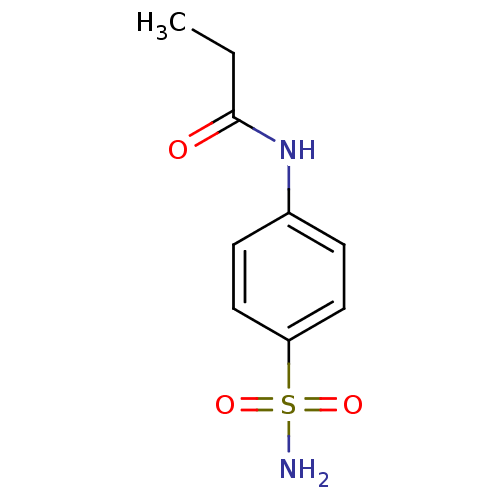 Purchase Purchase | Wt: 242.2 BDBM16646 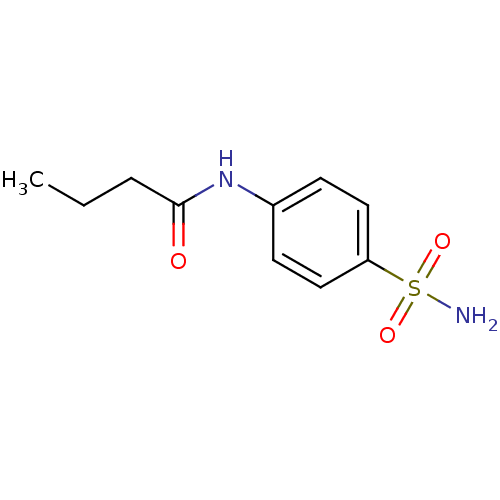 Purchase Purchase | Wt: 242.2 BDBM16647 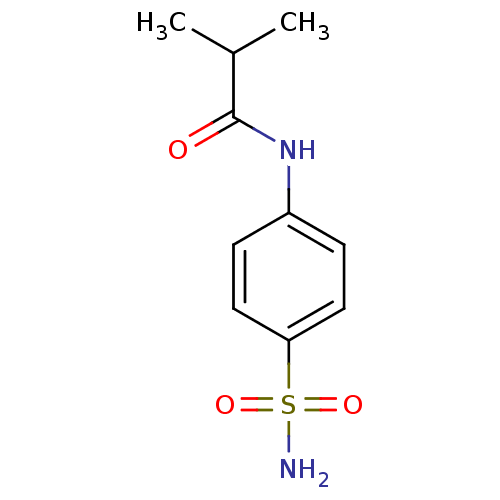 Purchase Purchase | Wt: 256.3 BDBM16648 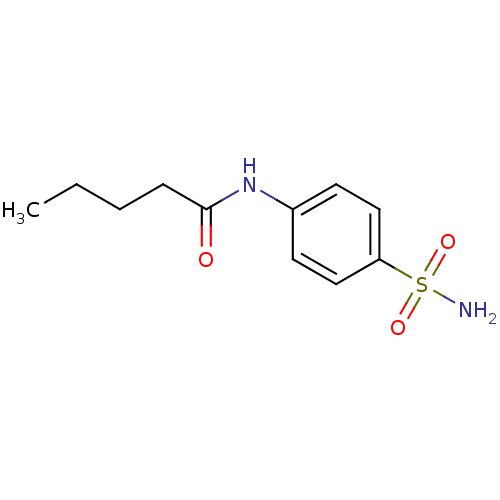 Purchase Purchase |
| << First | Previous | Displayed 16 to 30 (of 31 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16662 (1-N-(4-nitrobenzene)benzene-1,4-disulfonamide | ar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Bioscienze e Biorisorse Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins followed by CO2 addition measured for 10 to ... | Bioorg Med Chem Lett 27: 490-495 (2017) Article DOI: 10.1016/j.bmcl.2016.12.035 BindingDB Entry DOI: 10.7270/Q2PN97XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16644 (2,2,2-trifluoro-N-(4-sulfamoylphenyl)acetamide | 4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16644 (2,2,2-trifluoro-N-(4-sulfamoylphenyl)acetamide | 4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase II (hCA II) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM16643 (4-Acetamido-benzenesulfonamide | CHEMBL687 | N-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase IX (hCA IX) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM16647 (2-methyl-N-(4-sulfamoylphenyl)propanamide | 4-Isob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase XII (hCA XII) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM16647 (2-methyl-N-(4-sulfamoylphenyl)propanamide | 4-Isob...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase IX (hCA IX) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM16648 (4-n-Pentanamido-benzenesulfonamide | CHEMBL283097 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase XII (hCA XII) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16663 (N-(4-{[2-(4-sulfamoylphenyl)ethyl]sulfamoyl}phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 5A, mitochondrial (Mus musculus (mouse)) | BDBM16646 (4-n-Butanamido-benzenesulfonamide | CHEMBL23591 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 5A, mitochondrial (Mus musculus (mouse)) | BDBM16645 (4-Propionamido-benzenesulfonamide | CHEMBL278395 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 5A, mitochondrial (Mus musculus (mouse)) | BDBM16643 (4-Acetamido-benzenesulfonamide | CHEMBL687 | N-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16648 (4-n-Pentanamido-benzenesulfonamide | CHEMBL283097 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16648 (4-n-Pentanamido-benzenesulfonamide | CHEMBL283097 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase II (hCA II) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16646 (4-n-Butanamido-benzenesulfonamide | CHEMBL23591 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase II (hCA II) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16646 (4-n-Butanamido-benzenesulfonamide | CHEMBL23591 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16645 (4-Propionamido-benzenesulfonamide | CHEMBL278395 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16645 (4-Propionamido-benzenesulfonamide | CHEMBL278395 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase II (hCA II) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 5A, mitochondrial (Mus musculus (mouse)) | BDBM16647 (2-methyl-N-(4-sulfamoylphenyl)propanamide | 4-Isob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16643 (4-Acetamido-benzenesulfonamide | CHEMBL687 | N-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16643 (4-Acetamido-benzenesulfonamide | CHEMBL687 | N-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase II (hCA II) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16647 (2-methyl-N-(4-sulfamoylphenyl)propanamide | 4-Isob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase II (hCA II) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM16647 (2-methyl-N-(4-sulfamoylphenyl)propanamide | 4-Isob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM16661 (1-N-(4-fluorobenzene)benzene-1,4-disulfonamide | a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM16644 (2,2,2-trifluoro-N-(4-sulfamoylphenyl)acetamide | 4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM16648 (4-n-Pentanamido-benzenesulfonamide | CHEMBL283097 ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM16646 (4-n-Butanamido-benzenesulfonamide | CHEMBL23591 | ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM16645 (4-Propionamido-benzenesulfonamide | CHEMBL278395 |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM16647 (2-methyl-N-(4-sulfamoylphenyl)propanamide | 4-Isob...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM16643 (4-Acetamido-benzenesulfonamide | CHEMBL687 | N-(4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16644 (2,2,2-trifluoro-N-(4-sulfamoylphenyl)acetamide | 4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.46E+4 | -6.59 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16644 (2,2,2-trifluoro-N-(4-sulfamoylphenyl)acetamide | 4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase I (hCA I) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16648 (4-n-Pentanamido-benzenesulfonamide | CHEMBL283097 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+4 | -6.48 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16648 (4-n-Pentanamido-benzenesulfonamide | CHEMBL283097 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase I (hCA I) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16646 (4-n-Butanamido-benzenesulfonamide | CHEMBL23591 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase I (hCA I) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16646 (4-n-Butanamido-benzenesulfonamide | CHEMBL23591 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.93E+4 | -6.43 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16645 (4-Propionamido-benzenesulfonamide | CHEMBL278395 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase I (hCA I) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16645 (4-Propionamido-benzenesulfonamide | CHEMBL278395 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.97E+4 | -6.41 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16662 (1-N-(4-nitrobenzene)benzene-1,4-disulfonamide | ar...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Bioscienze e Biorisorse Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 assessed as reduction in CO2 hydration preincubated for 15 mins followed by CO2 addition measure... | Bioorg Med Chem Lett 27: 490-495 (2017) Article DOI: 10.1016/j.bmcl.2016.12.035 BindingDB Entry DOI: 10.7270/Q2PN97XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16643 (4-Acetamido-benzenesulfonamide | CHEMBL687 | N-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase I (hCA I) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16643 (4-Acetamido-benzenesulfonamide | CHEMBL687 | N-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.14E+4 | -6.37 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16647 (2-methyl-N-(4-sulfamoylphenyl)propanamide | 4-Isob...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.35E+4 | -6.31 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM16647 (2-methyl-N-(4-sulfamoylphenyl)propanamide | 4-Isob...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase I (hCA I) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM16661 (1-N-(4-fluorobenzene)benzene-1,4-disulfonamide | a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine carbonic anhydrase 2 assessed as hydrolysis of p-nitrophenyl acetate after 30 mins by spectrophotometric method | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM16661 (1-N-(4-fluorobenzene)benzene-1,4-disulfonamide | a...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of calf intestinal alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectrophotometric... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM16661 (1-N-(4-fluorobenzene)benzene-1,4-disulfonamide | a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| << First | Previous | Displayed 51 to 95 (of 95 total ) |