 Found 5107 hits for monomerid = 50326053,50331096,50332294,50358430,50355494,50355495,50355496,50355497,50355500,50355501,50362058,50375646,50382959,50377352,50380246
Found 5107 hits for monomerid = 50326053,50331096,50332294,50358430,50355494,50355495,50355496,50355497,50355500,50355501,50362058,50375646,50382959,50377352,50380246 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK1 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (828-1132) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 (837-1142) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331096
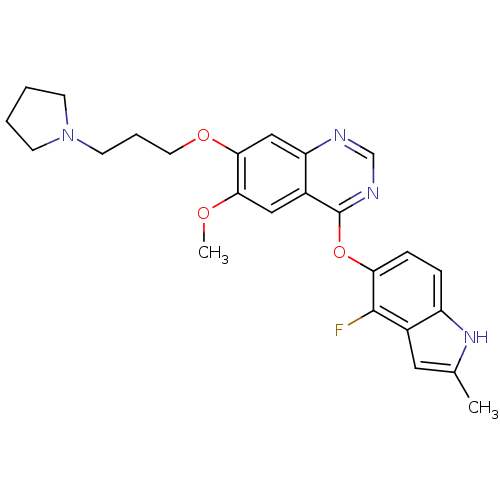
(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-6-methoxy-7...)Show SMILES COc1cc2c(Oc3ccc4[nH]c(C)cc4c3F)ncnc2cc1OCCCN1CCCC1 Show InChI InChI=1S/C25H27FN4O3/c1-16-12-17-19(29-16)6-7-21(24(17)26)33-25-18-13-22(31-2)23(14-20(18)27-15-28-25)32-11-5-10-30-8-3-4-9-30/h6-7,12-15,29H,3-5,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ontogenesis, LLC
US Patent
| Assay Description
Compounds 2-4 and cabozantinib were each tested for binding of c-Met, VEGFR2, TIE2 and the control compound, staurosporine. Specifically, each compou... |
US Patent US10464902 (2019)
BindingDB Entry DOI: 10.7270/Q2R213S2 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified TYK2 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50355500
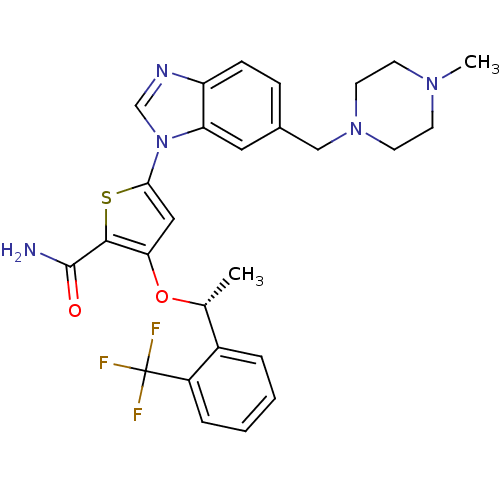
(CHEMBL1908394 | US9695172, GSK461364)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(CN3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N5O2S/c1-17(19-5-3-4-6-20(19)27(28,29)30)37-23-14-24(38-25(23)26(31)36)35-16-32-21-8-7-18(13-22(21)35)15-34-11-9-33(2)10-12-34/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H2,31,36)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127329
BindingDB Entry DOI: 10.7270/Q2Q243TB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50355500

(CHEMBL1908394 | US9695172, GSK461364)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(CN3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N5O2S/c1-17(19-5-3-4-6-20(19)27(28,29)30)37-23-14-24(38-25(23)26(31)36)35-16-32-21-8-7-18(13-22(21)35)15-34-11-9-33(2)10-12-34/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H2,31,36)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50355500

(CHEMBL1908394 | US9695172, GSK461364)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(CN3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N5O2S/c1-17(19-5-3-4-6-20(19)27(28,29)30)37-23-14-24(38-25(23)26(31)36)35-16-32-21-8-7-18(13-22(21)35)15-34-11-9-33(2)10-12-34/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H2,31,36)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His6-tagged PLK1 (1 to 603 residues) using casein as substrate preincubated for 60 mins followed by polo box peptide ... |
J Med Chem 60: 7863-7875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00996
BindingDB Entry DOI: 10.7270/Q26112G9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 kinase domain using N-terminal 5-carboxyfluorescein-tagged Leu-Pro-Leu-Asp-Lys-Asp-Tyr-Tyr-Val-Val-Arg as substrate after 30 mins |
J Med Chem 55: 6176-93 (2012)
Article DOI: 10.1021/jm300628c
BindingDB Entry DOI: 10.7270/Q25Q4X6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (781-1124) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of purified JAK3 incubated for 30 mins |
J Med Chem 55: 10090-107 (2012)
Article DOI: 10.1021/jm3012239
BindingDB Entry DOI: 10.7270/Q2Q241D4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50332294
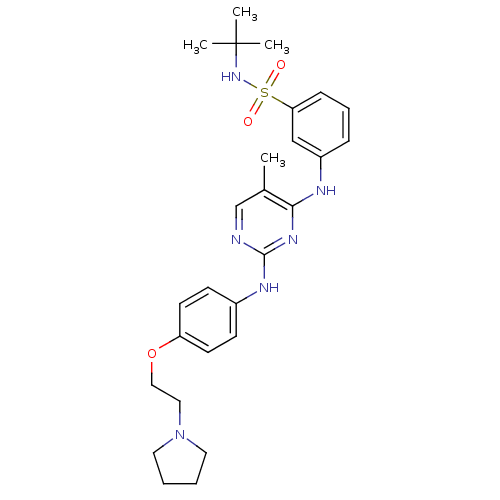
(CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(...)Show SMILES Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 |
J Med Chem 61: 9030-9058 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00667
BindingDB Entry DOI: 10.7270/Q2BK1GN8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50332294

(CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(...)Show SMILES Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human TYK2 |
J Med Chem 61: 9030-9058 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00667
BindingDB Entry DOI: 10.7270/Q2BK1GN8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50332294

(CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(...)Show SMILES Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 |
J Med Chem 61: 9030-9058 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00667
BindingDB Entry DOI: 10.7270/Q2BK1GN8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... |
Eur J Med Chem 178: 458-467 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.011
BindingDB Entry DOI: 10.7270/Q2BC42WH |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50382959
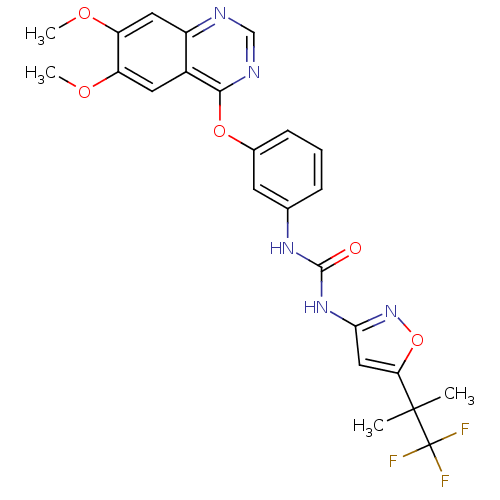
(CEP-32496 | CHEMBL2029988 | US9730937, Example 261)Show SMILES COc1cc2ncnc(Oc3cccc(NC(=O)Nc4cc(on4)C(C)(C)C(F)(F)F)c3)c2cc1OC Show InChI InChI=1S/C24H22F3N5O5/c1-23(2,24(25,26)27)19-11-20(32-37-19)31-22(33)30-13-6-5-7-14(8-13)36-21-15-9-17(34-3)18(35-4)10-16(15)28-12-29-21/h5-12H,1-4H3,(H2,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50382959

(CEP-32496 | CHEMBL2029988 | US9730937, Example 261)Show SMILES COc1cc2ncnc(Oc3cccc(NC(=O)Nc4cc(on4)C(C)(C)C(F)(F)F)c3)c2cc1OC Show InChI InChI=1S/C24H22F3N5O5/c1-23(2,24(25,26)27)19-11-20(32-37-19)31-22(33)30-13-6-5-7-14(8-13)36-21-15-9-17(34-3)18(35-4)10-16(15)28-12-29-21/h5-12H,1-4H3,(H2,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50355500

(CHEMBL1908394 | US9695172, GSK461364)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(CN3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N5O2S/c1-17(19-5-3-4-6-20(19)27(28,29)30)37-23-14-24(38-25(23)26(31)36)35-16-32-21-8-7-18(13-22(21)35)15-34-11-9-33(2)10-12-34/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H2,31,36)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Dana-Farber Cancer Institute, Inc.
US Patent
| Assay Description
Assays were performed with minor modifications from the manufacturer's protocol (PerkinElmer, USA). All reagents were diluted in 50 mM HEPES, 150 mM ... |
US Patent US9695172 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4TX8 |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein
(Homo sapiens (Human)) | BDBM50355500

(CHEMBL1908394 | US9695172, GSK461364)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(CN3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N5O2S/c1-17(19-5-3-4-6-20(19)27(28,29)30)37-23-14-24(38-25(23)26(31)36)35-16-32-21-8-7-18(13-22(21)35)15-34-11-9-33(2)10-12-34/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H2,31,36)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Dana-Farber Cancer Institute, Inc.
US Patent
| Assay Description
Assays were performed with minor modifications from the manufacturer's protocol (PerkinElmer, USA). All reagents were diluted in 50 mM HEPES, 150 mM ... |
US Patent US9695172 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4TX8 |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50380246
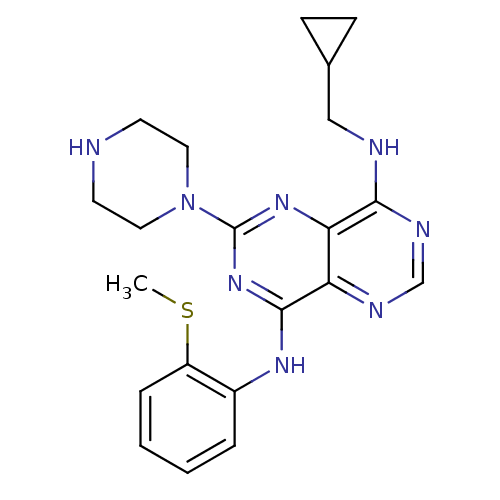
(CHEMBL2017214)Show SMILES CSc1ccccc1Nc1nc(nc2c(NCC3CC3)ncnc12)N1CCNCC1 Show InChI InChI=1S/C21H26N8S/c1-30-16-5-3-2-4-15(16)26-20-17-18(19(25-13-24-17)23-12-14-6-7-14)27-21(28-20)29-10-8-22-9-11-29/h2-5,13-14,22H,6-12H2,1H3,(H,23,24,25)(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Companies of Johnson& Johnson
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human hepatic KHKC |
Bioorg Med Chem Lett 22: 5326-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.008
BindingDB Entry DOI: 10.7270/Q21J9BV6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin) |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2/Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1/TYK2 in human whole blood assessed as inhibition of IFN-alpha-induced STAT-3 phosphorylation preincubated for 45 mins followed by ... |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2/Tyrosine-protein kinase JAK1/Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1/JAK2/TYK2 in human whole blood assessed as inhibition of IL-6-induced STAT-1 phosphorylation preincubated for 45 mins followed by ... |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 487 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 677 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 homodimer in human CD34+ cells spiked into human whole blood assessed as inhibition of EPO-induced STAT-5 phosphorylation preincub... |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2/Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 818 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2/TYK2 in human whole blood assessed as inhibition of IL-23-induced STAT-3 phosphorylation preincubated for 45 mins followed by IL-2... |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2/Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2/TYK2 in human whole blood assessed as inhibition of IL-12-induced STAT-4 phosphorylation preincubated for 45 mins followed by IL-1... |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
JAK3/JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1/JAK3 in human whole blood assessed as inhibition of IL-15-induced STAT-5 phosphorylation preincubated for 45 mins followed by IL-1... |
J Med Chem 57: 5023-38 (2014)
Article DOI: 10.1021/jm401490p
BindingDB Entry DOI: 10.7270/Q2ZS2Z3X |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 1
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to EPHB1 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to HPK1 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to PDGFRA |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to p38-delta |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Homeodomain-interacting protein kinase 2
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to HIPK2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase FRK
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to FRK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 4
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to ERK4 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to FGFR4 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek2
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to NEK2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to MAP3K1 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK3
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to CLK3 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase RIO3
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to RIOK3 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to PRKCD |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK7
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to GRK7 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to GSK3B |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to IGF1R |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase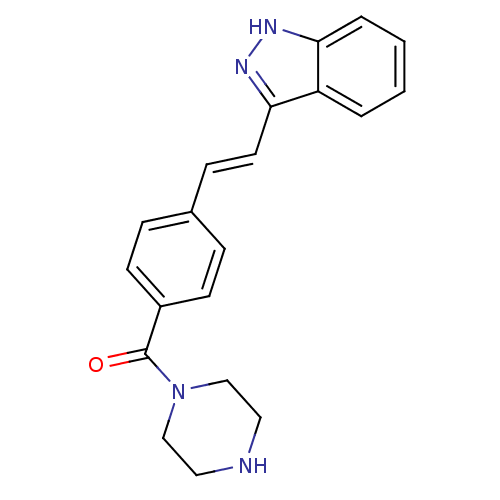 Purchase
Purchase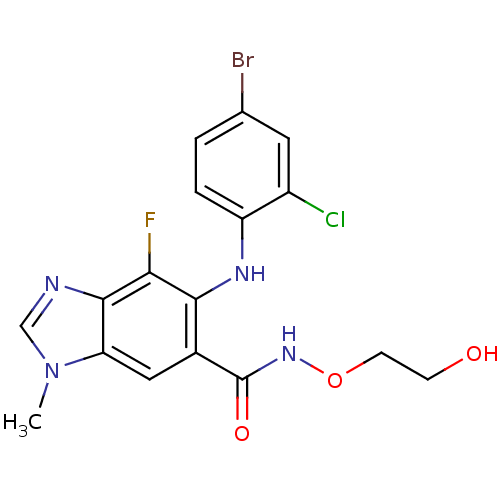 Purchase
Purchase Purchase
Purchase Purchase
Purchase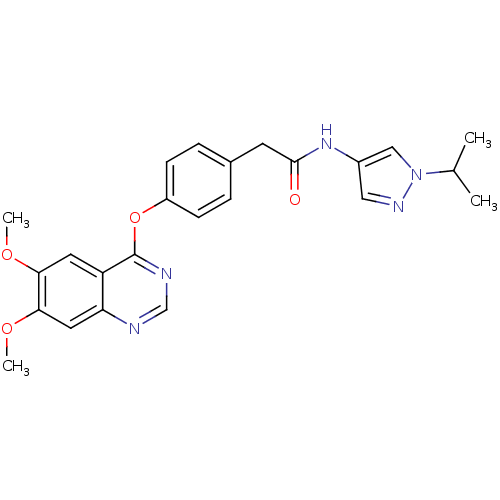 Purchase
Purchase Purchase
Purchase Purchase
Purchase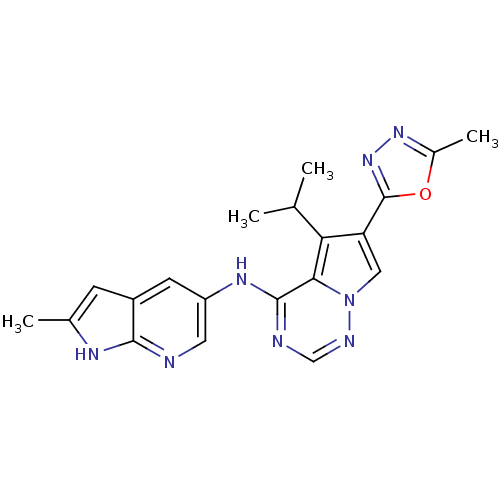 Purchase
Purchase Purchase
Purchase