 Found 79 hits for monomerid = 50442299,50444549,50445346,50447470,50447471
Found 79 hits for monomerid = 50442299,50444549,50445346,50447470,50447471 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin D2 receptor 2
(Mus musculus (mouse)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prostaglandin D2 from mouse CRTh2 receptor expressed in CHO cells after 2 hrs |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50445346
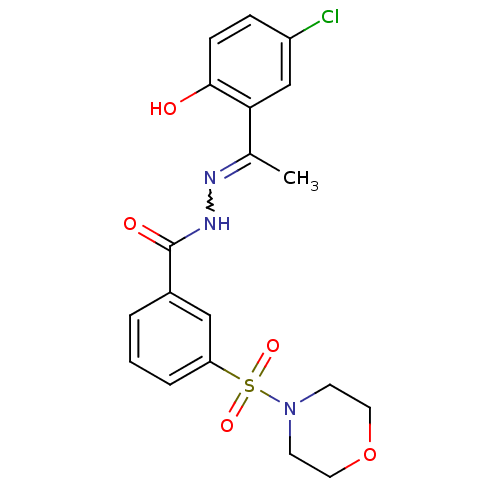
(CHEMBL3104250)Show SMILES CC(=NNC(=O)c1cccc(c1)S(=O)(=O)N1CCOCC1)c1cc(Cl)ccc1O |w:2.2| Show InChI InChI=1S/C19H20ClN3O5S/c1-13(17-12-15(20)5-6-18(17)24)21-22-19(25)14-3-2-4-16(11-14)29(26,27)23-7-9-28-10-8-23/h2-6,11-12,24H,7-10H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 1 ... |
J Med Chem 56: 9496-508 (2014)
Article DOI: 10.1021/jm400870h
BindingDB Entry DOI: 10.7270/Q2Z60QJ7 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50445346

(CHEMBL3104250)Show SMILES CC(=NNC(=O)c1cccc(c1)S(=O)(=O)N1CCOCC1)c1cc(Cl)ccc1O |w:2.2| Show InChI InChI=1S/C19H20ClN3O5S/c1-13(17-12-15(20)5-6-18(17)24)21-22-19(25)14-3-2-4-16(11-14)29(26,27)23-7-9-28-10-8-23/h2-6,11-12,24H,7-10H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 10... |
J Med Chem 56: 9496-508 (2014)
Article DOI: 10.1021/jm400870h
BindingDB Entry DOI: 10.7270/Q2Z60QJ7 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50445346

(CHEMBL3104250)Show SMILES CC(=NNC(=O)c1cccc(c1)S(=O)(=O)N1CCOCC1)c1cc(Cl)ccc1O |w:2.2| Show InChI InChI=1S/C19H20ClN3O5S/c1-13(17-12-15(20)5-6-18(17)24)21-22-19(25)14-3-2-4-16(11-14)29(26,27)23-7-9-28-10-8-23/h2-6,11-12,24H,7-10H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 3 ... |
J Med Chem 56: 9496-508 (2014)
Article DOI: 10.1021/jm400870h
BindingDB Entry DOI: 10.7270/Q2Z60QJ7 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50445346

(CHEMBL3104250)Show SMILES CC(=NNC(=O)c1cccc(c1)S(=O)(=O)N1CCOCC1)c1cc(Cl)ccc1O |w:2.2| Show InChI InChI=1S/C19H20ClN3O5S/c1-13(17-12-15(20)5-6-18(17)24)21-22-19(25)14-3-2-4-16(11-14)29(26,27)23-7-9-28-10-8-23/h2-6,11-12,24H,7-10H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 30... |
J Med Chem 56: 9496-508 (2014)
Article DOI: 10.1021/jm400870h
BindingDB Entry DOI: 10.7270/Q2Z60QJ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human DP2 receptor expressed in CHO cell membranes after 60 mins by scintillation proximity assay |
ACS Med Chem Lett 8: 582-586 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00157
BindingDB Entry DOI: 10.7270/Q2TH8PXN |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50445346

(CHEMBL3104250)Show SMILES CC(=NNC(=O)c1cccc(c1)S(=O)(=O)N1CCOCC1)c1cc(Cl)ccc1O |w:2.2| Show InChI InChI=1S/C19H20ClN3O5S/c1-13(17-12-15(20)5-6-18(17)24)21-22-19(25)14-3-2-4-16(11-14)29(26,27)23-7-9-28-10-8-23/h2-6,11-12,24H,7-10H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 10... |
J Med Chem 56: 9496-508 (2014)
Article DOI: 10.1021/jm400870h
BindingDB Entry DOI: 10.7270/Q2Z60QJ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRTh2 receptor expressed in CHO cells assessed as inhibition of prostaglandin D2 and forskolin-induced cAMP accumulation... |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor in human isolated eosinophil assessed as inhibition of DK-PGD2-induced shape change after 5 mins by flow cytome... |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of prostanoid EP4 receptor (unknown origin) |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor in human isolated eosinophil assessed as inhibition of eotaxin-induced shape change after 5 mins by flow cytome... |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor in human whole blood assessed as inhibition of DK-PGD2-induced eosinophil shape change after 5 mins by flow cyt... |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of prostanoid EP3 receptor (unknown origin) |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor in human whole blood assessed as inhibition of PGD2-induced eosinophil shape change after 5 mins by flow cytome... |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor in human isolated eosinophil assessed as inhibition of 11-Dehydro-TXB2-induced shape change after 5 mins by flo... |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor in human isolated eosinophil assessed as inhibition of delta12-PGD2-induced shape change after 5 mins by flow c... |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of prostanoid EP2 receptor (unknown origin) |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster V79MZh cells using deoxycorticosterone as substrate |
J Med Chem 57: 5179-89 (2014)
Article DOI: 10.1021/jm500140c
BindingDB Entry DOI: 10.7270/Q20Z74VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79MZh cells using deoxycorticosterone as substrate |
J Med Chem 57: 5179-89 (2014)
Article DOI: 10.1021/jm500140c
BindingDB Entry DOI: 10.7270/Q20Z74VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 (unknown origin) |
J Med Chem 57: 5179-89 (2014)
Article DOI: 10.1021/jm500140c
BindingDB Entry DOI: 10.7270/Q20Z74VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS)
Curated by ChEMBL
| Assay Description
Inhibition of human aldosterone synthase expressed in V79 MZ cells |
J Med Chem 57: 5011-22 (2014)
Article DOI: 10.1021/jm401430e
BindingDB Entry DOI: 10.7270/Q2QR4ZRP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 856 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS)
Curated by ChEMBL
| Assay Description
Inhibition of CYP19 (unknown origin) |
J Med Chem 57: 5011-22 (2014)
Article DOI: 10.1021/jm401430e
BindingDB Entry DOI: 10.7270/Q2QR4ZRP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS)
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in V79 MZ cells |
J Med Chem 57: 5011-22 (2014)
Article DOI: 10.1021/jm401430e
BindingDB Entry DOI: 10.7270/Q2QR4ZRP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 in human V79MZ cells using [3H]-11-deoxycorticosterone as substrate incubated for 1 hr prior to substrate addition measured aft... |
Eur J Med Chem 90: 788-96 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.022
BindingDB Entry DOI: 10.7270/Q2PZ5BGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B1 in human V79MZ cells using [3H]-11-deoxycorticosterone as substrate incubated for 1 hr prior to substrate addition measured aft... |
Eur J Med Chem 90: 788-96 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.022
BindingDB Entry DOI: 10.7270/Q2PZ5BGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS)
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method |
J Med Chem 58: 2530-7 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00079
BindingDB Entry DOI: 10.7270/Q20003S5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS)
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 MZh cells using [14C]-deoxycorticosterone substrate incubated for 6 hrs by HPTLC method |
J Med Chem 58: 2530-7 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00079
BindingDB Entry DOI: 10.7270/Q20003S5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in renal leiomyoblastoma cells |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in renal leiomyoblastoma cells |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Rattus norvegicus) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 495 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP19 preincubated for 10 mins followed by protein addition measured after 90 mins by fluorimetric analysis |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447470

(CHEMBL3115180)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447471
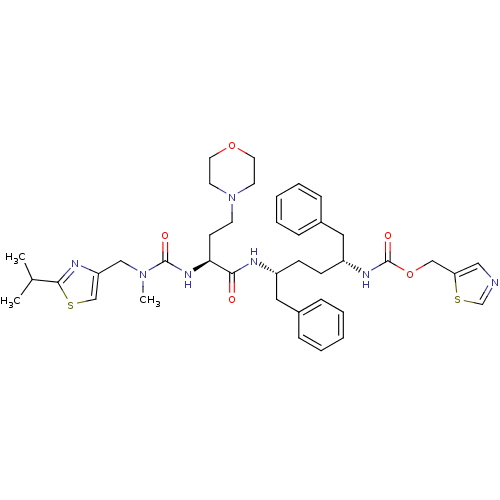
(CHEBI:72291 | Cobicistat)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50447470

(CHEMBL3115180)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50447471

(CHEBI:72291 | Cobicistat)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447470

(CHEMBL3115180)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447471

(CHEBI:72291 | Cobicistat)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50447470

(CHEMBL3115180)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50447471

(CHEBI:72291 | Cobicistat)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of prostanoid TP receptor (unknown origin) |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of prostanoid IP receptor (unknown origin) |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of prostanoid FP receptor (unknown origin) |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase