 Found 14 hits in this display
Found 14 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 5
(Chicken) | BDBM50089038
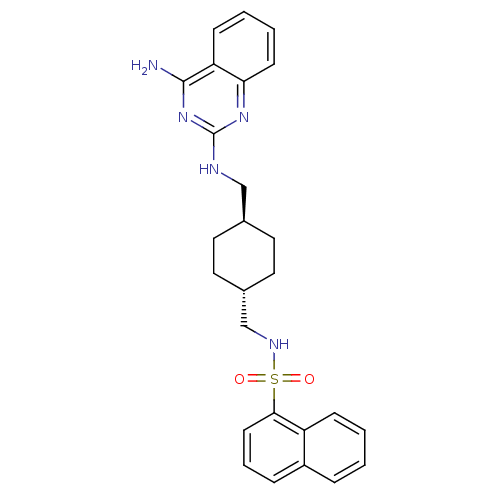
(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by PDSP Ki Database
| |
J Neurochem 81: 462-71 (2002)
Article DOI: 10.1046/j.1471-4159.2002.00817.x
BindingDB Entry DOI: 10.7270/Q2T72G1G |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Chicken) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by PDSP Ki Database
| |
J Neurochem 81: 462-71 (2002)
Article DOI: 10.1046/j.1471-4159.2002.00817.x
BindingDB Entry DOI: 10.7270/Q2T72G1G |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50166561
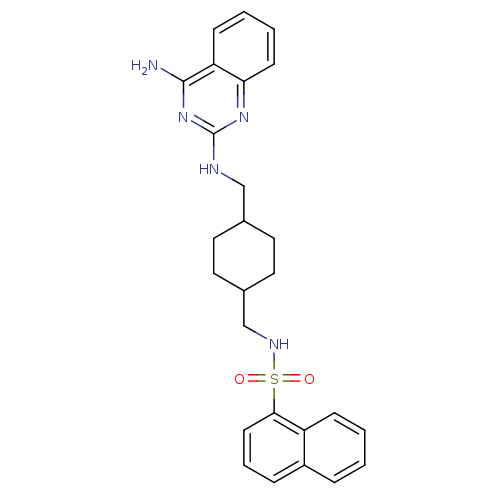
(CHEMBL195380 | Naphthalene-1-sulfonic acid {4-[(4-...)Show SMILES Nc1nc(NCC2CCC(CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |(-3.12,3.75,;-3.12,2.21,;-1.77,1.44,;-1.75,-.12,;-.44,-.89,;.91,-.12,;2.24,-.9,;3.57,-.12,;4.91,-.91,;4.89,-2.45,;6.22,-3.23,;7.57,-2.46,;8.9,-3.25,;9.65,-1.9,;7.79,-4.33,;10.21,-4.05,;11.56,-3.32,;12.87,-4.12,;12.83,-5.66,;11.48,-6.4,;11.43,-7.93,;10.09,-8.67,;8.77,-7.86,;8.81,-6.31,;10.17,-5.59,;3.56,-3.21,;2.23,-2.44,;-3.1,-.9,;-4.45,-.12,;-5.78,-.9,;-7.12,-.12,;-7.12,1.42,;-5.78,2.19,;-4.45,1.42,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY |
Bioorg Med Chem Lett 15: 2565-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.052
BindingDB Entry DOI: 10.7270/Q2Z89BXQ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Compound was tested for rat Neuropeptide Y receptor type 5 |
Bioorg Med Chem Lett 10: 1175-9 (2000)
BindingDB Entry DOI: 10.7270/Q2KH0MJP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Compound was tested for human Neuropeptide Y receptor type 5 |
Bioorg Med Chem Lett 10: 1175-9 (2000)
BindingDB Entry DOI: 10.7270/Q2KH0MJP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Compound was tested for its antagonistic activity against Neuropeptide Y receptor Y5 subtype stably expressed in LM(tk-)cells |
Bioorg Med Chem Lett 10: 1175-9 (2000)
BindingDB Entry DOI: 10.7270/Q2KH0MJP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Navarra
Curated by ChEMBL
| Assay Description
Human Neuropeptide Y5 receptor binding affinity |
Bioorg Med Chem Lett 14: 597-9 (2004)
BindingDB Entry DOI: 10.7270/Q2513XMW |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50166561

(CHEMBL195380 | Naphthalene-1-sulfonic acid {4-[(4-...)Show SMILES Nc1nc(NCC2CCC(CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |(-3.12,3.75,;-3.12,2.21,;-1.77,1.44,;-1.75,-.12,;-.44,-.89,;.91,-.12,;2.24,-.9,;3.57,-.12,;4.91,-.91,;4.89,-2.45,;6.22,-3.23,;7.57,-2.46,;8.9,-3.25,;9.65,-1.9,;7.79,-4.33,;10.21,-4.05,;11.56,-3.32,;12.87,-4.12,;12.83,-5.66,;11.48,-6.4,;11.43,-7.93,;10.09,-8.67,;8.77,-7.86,;8.81,-6.31,;10.17,-5.59,;3.56,-3.21,;2.23,-2.44,;-3.1,-.9,;-4.45,-.12,;-5.78,-.9,;-7.12,-.12,;-7.12,1.42,;-5.78,2.19,;-4.45,1.42,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 |
Bioorg Med Chem Lett 15: 2565-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.052
BindingDB Entry DOI: 10.7270/Q2Z89BXQ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Compound was tested for human Neuropeptide Y receptor type 2 |
Bioorg Med Chem Lett 10: 1175-9 (2000)
BindingDB Entry DOI: 10.7270/Q2KH0MJP |
More data for this
Ligand-Target Pair | |
Aquaporin-1
(Homo sapiens (Human)) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Institute
| Assay Description
Osmotic water permeability was measured at 22-24 °C bymonitoring 90° scattered light intensity at 520 nm wavelength. Measurements were made using a P... |
Chem Biol Drug Des 87: 794-805 (2016)
Article DOI: 10.1111/cbdd.12713
BindingDB Entry DOI: 10.7270/Q2GQ6WHZ |
More data for this
Ligand-Target Pair | |
Aquaporin-1
(Homo sapiens (Human)) | BDBM50166561

(CHEMBL195380 | Naphthalene-1-sulfonic acid {4-[(4-...)Show SMILES Nc1nc(NCC2CCC(CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |(-3.12,3.75,;-3.12,2.21,;-1.77,1.44,;-1.75,-.12,;-.44,-.89,;.91,-.12,;2.24,-.9,;3.57,-.12,;4.91,-.91,;4.89,-2.45,;6.22,-3.23,;7.57,-2.46,;8.9,-3.25,;9.65,-1.9,;7.79,-4.33,;10.21,-4.05,;11.56,-3.32,;12.87,-4.12,;12.83,-5.66,;11.48,-6.4,;11.43,-7.93,;10.09,-8.67,;8.77,-7.86,;8.81,-6.31,;10.17,-5.59,;3.56,-3.21,;2.23,-2.44,;-3.1,-.9,;-4.45,-.12,;-5.78,-.9,;-7.12,-.12,;-7.12,1.42,;-5.78,2.19,;-4.45,1.42,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50166561

(CHEMBL195380 | Naphthalene-1-sulfonic acid {4-[(4-...)Show SMILES Nc1nc(NCC2CCC(CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |(-3.12,3.75,;-3.12,2.21,;-1.77,1.44,;-1.75,-.12,;-.44,-.89,;.91,-.12,;2.24,-.9,;3.57,-.12,;4.91,-.91,;4.89,-2.45,;6.22,-3.23,;7.57,-2.46,;8.9,-3.25,;9.65,-1.9,;7.79,-4.33,;10.21,-4.05,;11.56,-3.32,;12.87,-4.12,;12.83,-5.66,;11.48,-6.4,;11.43,-7.93,;10.09,-8.67,;8.77,-7.86,;8.81,-6.31,;10.17,-5.59,;3.56,-3.21,;2.23,-2.44,;-3.1,-.9,;-4.45,-.12,;-5.78,-.9,;-7.12,-.12,;-7.12,1.42,;-5.78,2.19,;-4.45,1.42,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... |
Bioorg Med Chem Lett 15: 2565-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.052
BindingDB Entry DOI: 10.7270/Q2Z89BXQ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Compound was tested for human Neuropeptide Y receptor type 4 |
Bioorg Med Chem Lett 10: 1175-9 (2000)
BindingDB Entry DOI: 10.7270/Q2KH0MJP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50089038

(CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12 |wU:6.5,wD:9.9,(2.92,.42,;2.94,-1.13,;4.28,-1.89,;4.29,-3.44,;5.63,-4.22,;6.96,-3.45,;8.29,-4.21,;9.63,-3.44,;10.96,-4.22,;10.95,-5.76,;12.28,-6.54,;13.77,-6.13,;14.86,-7.21,;13.98,-8.47,;15.7,-5.91,;16.12,-8.05,;17.24,-7,;18.73,-7.45,;19.09,-8.94,;17.97,-9.99,;18.33,-11.48,;17.22,-12.56,;15.73,-12.11,;15.38,-10.62,;16.49,-9.56,;9.62,-6.51,;8.29,-5.75,;2.95,-4.23,;1.61,-3.45,;.26,-4.23,;-1.07,-3.46,;-1.07,-1.9,;.26,-1.13,;1.61,-1.9,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-22-9-3-4-10-23(22)30-26(31-25)28-16-18-12-14-19(15-13-18)17-29-34(32,33)24-11-5-7-20-6-1-2-8-21(20)24/h1-11,18-19,29H,12-17H2,(H3,27,28,30,31)/t18-,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Compound was tested for human Neuropeptide Y receptor type 1 |
Bioorg Med Chem Lett 10: 1175-9 (2000)
BindingDB Entry DOI: 10.7270/Q2KH0MJP |
More data for this
Ligand-Target Pair | |