 Found 453 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein kinase Blk'
Found 453 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein kinase Blk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 32.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50515526
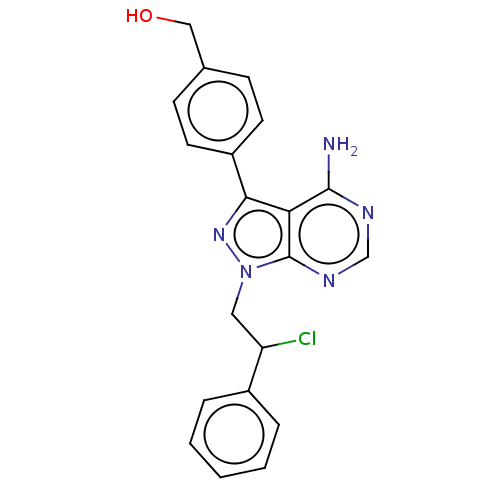
(CHEMBL4452230)Show SMILES Nc1ncnc2n(CC(Cl)c3ccccc3)nc(-c3ccc(CO)cc3)c12 Show InChI InChI=1S/C20H18ClN5O/c21-16(14-4-2-1-3-5-14)10-26-20-17(19(22)23-12-24-20)18(25-26)15-8-6-13(11-27)7-9-15/h1-9,12,16,27H,10-11H2,(H2,22,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of BLK (unknown origin) using KVEKIGEGTYGVVYK as substrate in presence of [gamma33P]ATP as substrate in presence of [gamma33P]ATP by scint... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.048
BindingDB Entry DOI: 10.7270/Q2B85CHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50089575

(CHEMBL3578214)Show SMILES Nc1ncnc2n(CC(Cl)c3ccccc3)nc(-c3ccc(Cl)cc3)c12 Show InChI InChI=1S/C19H15Cl2N5/c20-14-8-6-13(7-9-14)17-16-18(22)23-11-24-19(16)26(25-17)10-15(21)12-4-2-1-3-5-12/h1-9,11,15H,10H2,(H2,22,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of BLK (unknown origin) using KVEKIGEGTYGVVYK as substrate in presence of [gamma33P]ATP as substrate in presence of [gamma33P]ATP by scint... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.048
BindingDB Entry DOI: 10.7270/Q2B85CHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50402020
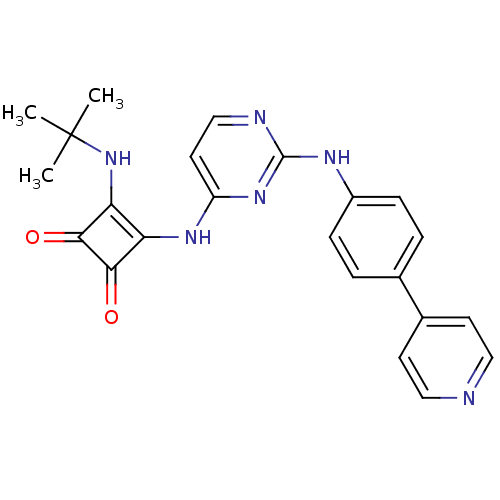
(CHEMBL2205426)Show SMILES CC(C)(C)Nc1c(Nc2ccnc(Nc3ccc(cc3)-c3ccncc3)n2)c(=O)c1=O Show InChI InChI=1S/C23H22N6O2/c1-23(2,3)29-19-18(20(30)21(19)31)27-17-10-13-25-22(28-17)26-16-6-4-14(5-7-16)15-8-11-24-12-9-15/h4-13,29H,1-3H3,(H2,25,26,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant BLK after 1 hr by scintillation counter analysis in presence of gamma-[33P]ATP |
Bioorg Med Chem Lett 22: 7615-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.009
BindingDB Entry DOI: 10.7270/Q2XK8GQ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50224883
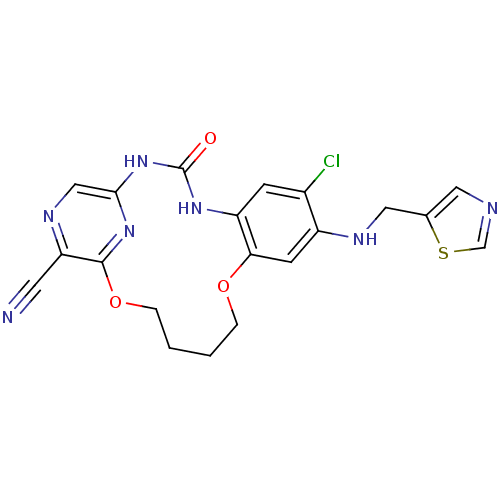
(7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...)Show SMILES Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-5-15-17(6-14(13)24-9-12-8-23-11-32-12)30-3-1-2-4-31-19-16(7-22)25-10-18(27-19)28-20(29)26-15/h5-6,8,10-11,24H,1-4,9H2,(H2,26,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BLK |
Bioorg Med Chem Lett 17: 6593-601 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.063
BindingDB Entry DOI: 10.7270/Q2X067WT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM13530
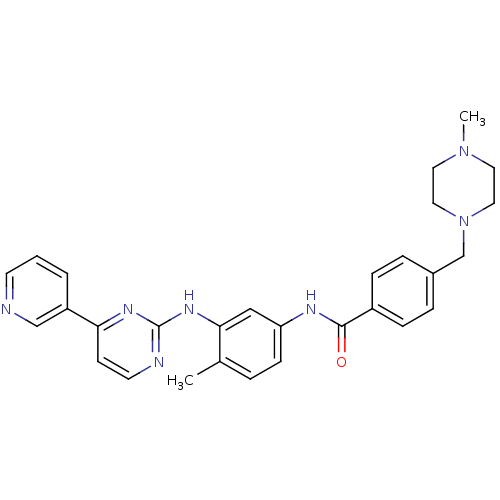
(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50463484
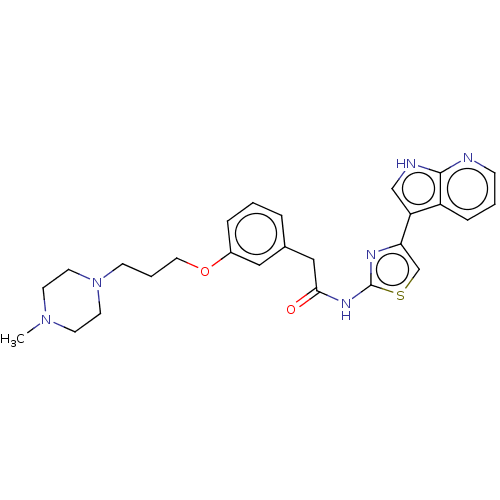
(CHEMBL4248525)Show SMILES CN1CCN(CCCOc2cccc(CC(=O)Nc3nc(cs3)-c3c[nH]c4ncccc34)c2)CC1 Show InChI InChI=1S/C26H30N6O2S/c1-31-10-12-32(13-11-31)9-4-14-34-20-6-2-5-19(15-20)16-24(33)30-26-29-23(18-35-26)22-17-28-25-21(22)7-3-8-27-25/h2-3,5-8,15,17-18H,4,9-14,16H2,1H3,(H,27,28)(H,29,30,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50237710
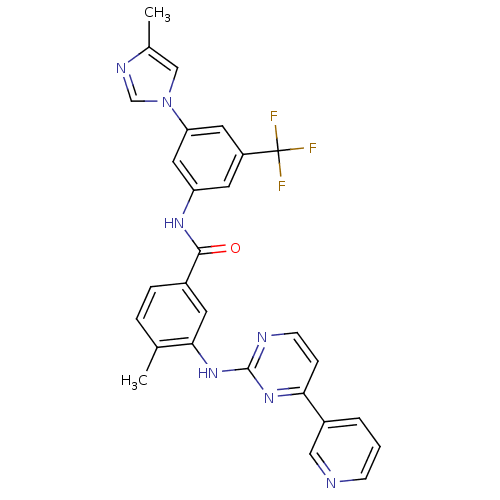
(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged cytoplasmic BLK expressed in baculovirus expression system by KT236 probe based TR-FRET assay |
J Med Chem 60: 1971-1993 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01694
BindingDB Entry DOI: 10.7270/Q2TX3JC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312
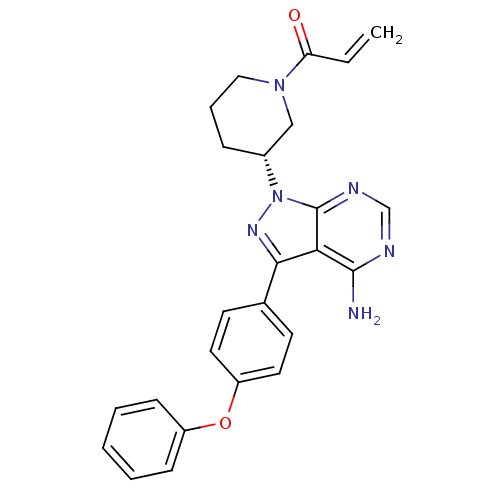
(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human His-tagged BLK cytoplasmic domain expressed in baculovirus expression system using tyrosine-1 peptide as ... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127261
BindingDB Entry DOI: 10.7270/Q2TB1BM3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114009
BindingDB Entry DOI: 10.7270/Q2ZK5MN1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM97672
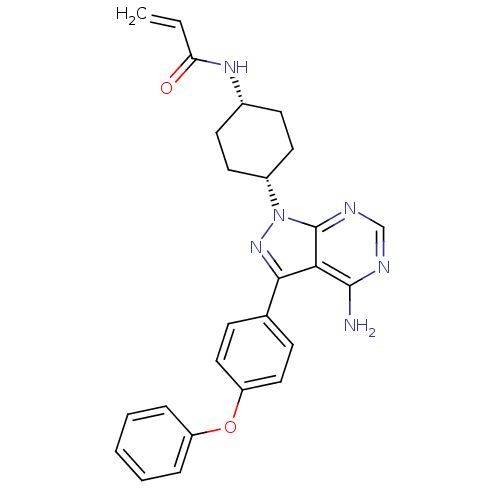
(US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NC(=O)C=C |r,wU:23.26,26.33,(-3.76,2.88,;-3.76,1.34,;-5.09,.57,;-5.09,-.97,;-3.76,-1.74,;-2.43,-.97,;-.96,-1.45,;-.06,-.2,;-.96,1.05,;-.56,2.53,;.92,2.93,;1.32,4.42,;.23,5.51,;.63,7,;2.12,7.4,;3.21,6.31,;4.7,6.7,;5.09,8.19,;4.01,9.28,;2.52,8.88,;-1.25,5.11,;-1.65,3.62,;-2.43,.57,;-.56,-2.93,;-1.65,-4.02,;-1.25,-5.51,;.23,-5.91,;1.32,-4.82,;.92,-3.33,;.63,-7.4,;2.12,-7.79,;2.52,-9.28,;3.21,-6.7,;4.7,-7.1,)| Show InChI InChI=1S/C26H26N6O2/c1-2-22(33)30-18-10-12-19(13-11-18)32-26-23(25(27)28-16-29-26)24(31-32)17-8-14-21(15-9-17)34-20-6-4-3-5-7-20/h2-9,14-16,18-19H,1,10-13H2,(H,30,33)(H2,27,28,29)/t18-,19+ | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... |
US Patent US9181263 (2015)
BindingDB Entry DOI: 10.7270/Q2765D5Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50455738

(CHEMBL4211949)Show SMILES Nc1ncnc2n3C[C@@H](NC(=O)C=C)C(=C)c3c(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C25H21N5O2/c1-3-20(31)29-19-13-30-23(15(19)2)21(22-24(26)27-14-28-25(22)30)16-9-11-18(12-10-16)32-17-7-5-4-6-8-17/h3-12,14,19H,1-2,13H2,(H,29,31)(H2,26,27,28)/t19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BLK using Poly(Glu, Tyr) 4:1 as substrate after 1 hr by ELISA |
J Med Chem 61: 4608-4627 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00441
BindingDB Entry DOI: 10.7270/Q2B85BRC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM97672

(US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NC(=O)C=C |r,wU:23.26,26.33,(-3.76,2.88,;-3.76,1.34,;-5.09,.57,;-5.09,-.97,;-3.76,-1.74,;-2.43,-.97,;-.96,-1.45,;-.06,-.2,;-.96,1.05,;-.56,2.53,;.92,2.93,;1.32,4.42,;.23,5.51,;.63,7,;2.12,7.4,;3.21,6.31,;4.7,6.7,;5.09,8.19,;4.01,9.28,;2.52,8.88,;-1.25,5.11,;-1.65,3.62,;-2.43,.57,;-.56,-2.93,;-1.65,-4.02,;-1.25,-5.51,;.23,-5.91,;1.32,-4.82,;.92,-3.33,;.63,-7.4,;2.12,-7.79,;2.52,-9.28,;3.21,-6.7,;4.7,-7.1,)| Show InChI InChI=1S/C26H26N6O2/c1-2-22(33)30-18-10-12-19(13-11-18)32-26-23(25(27)28-16-29-26)24(31-32)17-8-14-21(15-9-17)34-20-6-4-3-5-7-20/h2-9,14-16,18-19H,1,10-13H2,(H,30,33)(H2,27,28,29)/t18-,19+ | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... |
US Patent US9278100 (2016)
BindingDB Entry DOI: 10.7270/Q20C4TMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human BLK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m... |
J Med Chem 62: 7923-7940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00687
BindingDB Entry DOI: 10.7270/Q2RJ4NXF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human BLK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m... |
J Med Chem 62: 7923-7940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00687
BindingDB Entry DOI: 10.7270/Q2RJ4NXF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50589186
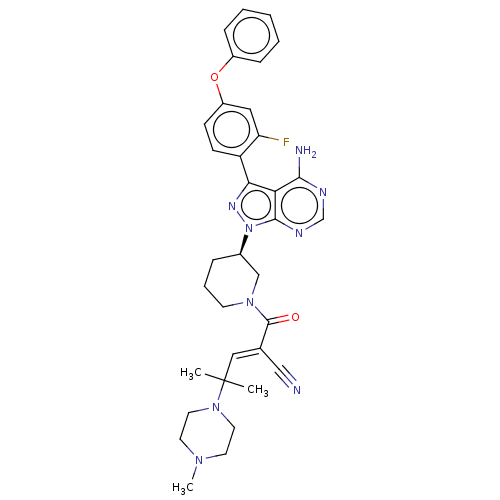
(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... |
US Patent US9181263 (2015)
BindingDB Entry DOI: 10.7270/Q2765D5Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... |
US Patent US9278100 (2016)
BindingDB Entry DOI: 10.7270/Q20C4TMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BLK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.12.079
BindingDB Entry DOI: 10.7270/Q2P272TR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BLK using Poly(Glu, Tyr) 4:1 as substrate after 1 hr by ELISA |
J Med Chem 61: 4608-4627 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00441
BindingDB Entry DOI: 10.7270/Q2B85BRC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BLK |
J Med Chem 55: 4539-50 (2012)
Article DOI: 10.1021/jm300035p
BindingDB Entry DOI: 10.7270/Q27H1KMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM538618

(NCGC 00262327 | US11254667, Compound I-20 | US1154...)Show SMILES COc1cc2ncc(-c3cccc(NC4CCNC4)n3)n2cc1-c1cn[nH]c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2154MW8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM538619
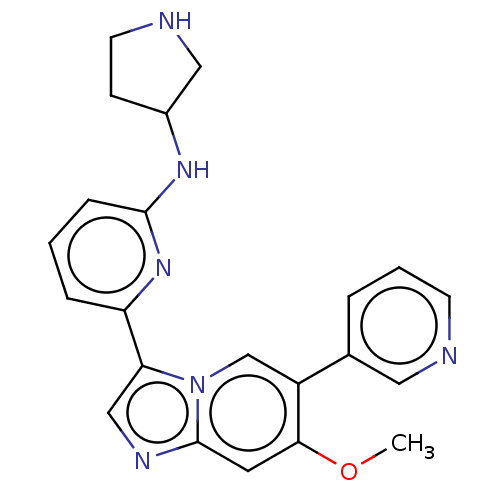
(NCGC 00371479 | US11254667, Compound I-22 | US1154...)Show SMILES COc1cc2ncc(-c3cccc(NC4CCNC4)n3)n2cc1-c1cccnc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2154MW8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM538621
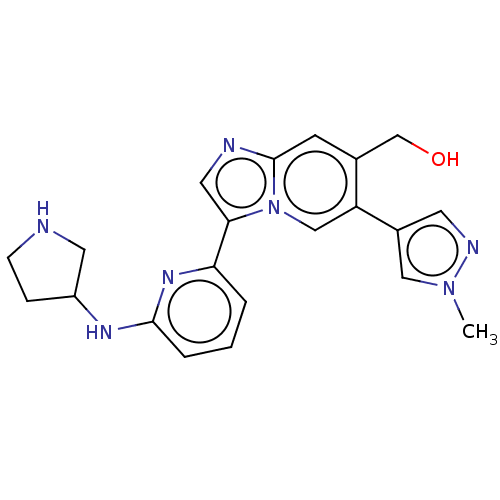
(NCGC 00371481 | US11254667, Compound I-24 | US1154...)Show SMILES Cn1cc(cn1)-c1cn2c(cnc2cc1CO)-c1cccc(NC2CCNC2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2154MW8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM139540
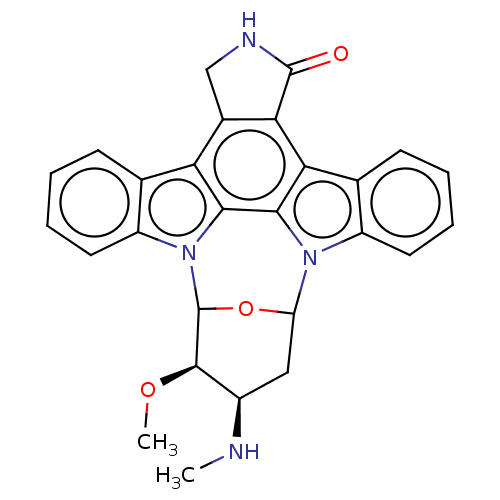
(US10189849, staurosporine | US10307427, Staurospor...)Show SMILES CN[C@@H]1CC2OC([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H24N4O3/c1-28-16-11-19-30-17-9-5-4-8-14(17)21-22-15(12-29-26(22)32)20-13-7-3-6-10-18(13)31(23(20)24(21)30)27(34-19)25(16)33-2/h3-10,16,19,25,27-28H,11-12H2,1-2H3,(H,29,32)/t16-,19?,25-,27?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2154MW8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM538619

(NCGC 00371479 | US11254667, Compound I-22 | US1154...)Show SMILES COc1cc2ncc(-c3cccc(NC4CCNC4)n3)n2cc1-c1cccnc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2154MW8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM538621

(NCGC 00371481 | US11254667, Compound I-24 | US1154...)Show SMILES Cn1cc(cn1)-c1cn2c(cnc2cc1CO)-c1cccc(NC2CCNC2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2154MW8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
HANGZHOU HERTZ PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Preparation of Compounds to be Tested:1) Using DMSO to prepare 50× compound stock solutions (same as the stock solution in Example 34) for later use;... |
US Patent US10711006 (2020)
BindingDB Entry DOI: 10.7270/Q2WH2T10 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50512346

(CHEMBL4464404)Show SMILES C=CC(=O)Nc1cccc(Oc2nc(Nc3ccc(cc3)N3CCOCC3)nc3[nH]cc(-c4ccncc4)c23)c1 Show InChI InChI=1S/C30H27N7O3/c1-2-26(38)33-22-4-3-5-24(18-22)40-29-27-25(20-10-12-31-13-11-20)19-32-28(27)35-30(36-29)34-21-6-8-23(9-7-21)37-14-16-39-17-15-37/h2-13,18-19H,1,14-17H2,(H,33,38)(H2,32,34,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of BLK (unknown origin) using STK as substrate by HTRF assay |
Eur J Med Chem 173: 167-183 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.055
BindingDB Entry DOI: 10.7270/Q2G1645G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human BLK using poly[Glu:Tyr](4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM250082

(US9447106, 27b (peak 2) | US9556188, Compound 27a)Show SMILES NC(=O)c1c2NCC[C@@H](C3CCN(CC3)C(=O)C=C)n2nc1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N5O3/c1-2-23(33)31-16-13-18(14-17-31)22-12-15-29-27-24(26(28)34)25(30-32(22)27)19-8-10-21(11-9-19)35-20-6-4-3-5-7-20/h2-11,18,22,29H,1,12-17H2,(H2,28,34)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human BLK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m... |
J Med Chem 62: 7923-7940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00687
BindingDB Entry DOI: 10.7270/Q2RJ4NXF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BLK using poly[Glu:Tyr](4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM250082

(US9447106, 27b (peak 2) | US9556188, Compound 27a)Show SMILES NC(=O)c1c2NCC[C@@H](C3CCN(CC3)C(=O)C=C)n2nc1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N5O3/c1-2-23(33)31-16-13-18(14-17-31)22-12-15-29-27-24(26(28)34)25(30-32(22)27)19-8-10-21(11-9-19)35-20-6-4-3-5-7-20/h2-11,18,22,29H,1,12-17H2,(H2,28,34)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human BLK using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m... |
J Med Chem 62: 7923-7940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00687
BindingDB Entry DOI: 10.7270/Q2RJ4NXF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BLK (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112339
BindingDB Entry DOI: 10.7270/Q2DF6VZ0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM452963

(US10711006, Compound I)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)nc3)c12)C1CCCN(C1)C(=O)C(Br)=C Show InChI InChI=1S/C24H22BrN7O2/c1-15(25)24(33)31-11-5-6-17(13-31)32-23-20(22(26)28-14-29-23)21(30-32)16-9-10-19(27-12-16)34-18-7-3-2-4-8-18/h2-4,7-10,12,14,17H,1,5-6,11,13H2,(H2,26,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
HANGZHOU HERTZ PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Preparation of Compounds to be Tested:1) Using DMSO to prepare 50× compound stock solutions (same as the stock solution in Example 34) for later use;... |
US Patent US10711006 (2020)
BindingDB Entry DOI: 10.7270/Q2WH2T10 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50594746

(CHEMBL5193128)Show SMILES NC(=O)C1C2NCC[C@@H](C3CCN(CC3)C(=O)C=C)N2N=C1c1ccc(Oc2ccccc2)cc1 |r,c:22| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114009
BindingDB Entry DOI: 10.7270/Q2ZK5MN1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
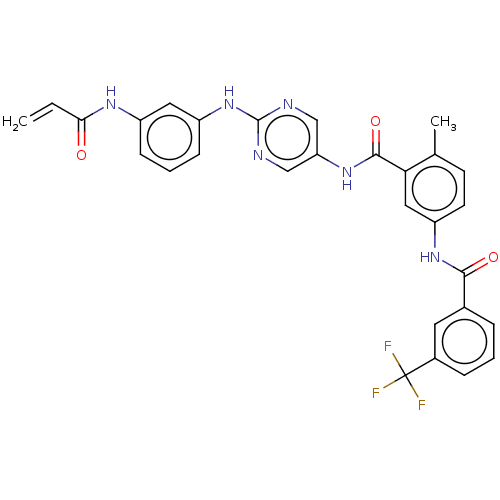
(Homo sapiens (Human)) | BDBM50020471
(CHEMBL3290142)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(NC(=O)C=C)c2)nc1 Show InChI InChI=1S/C29H23F3N6O3/c1-3-25(39)35-20-8-5-9-21(13-20)38-28-33-15-23(16-34-28)37-27(41)24-14-22(11-10-17(24)2)36-26(40)18-6-4-7-19(12-18)29(30,31)32/h3-16H,1H2,2H3,(H,35,39)(H,36,40)(H,37,41)(H,33,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
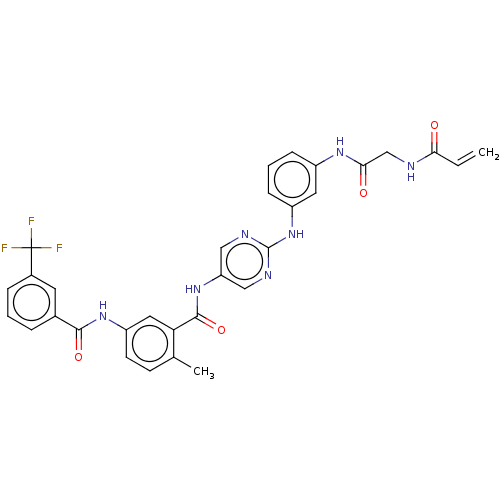
(Homo sapiens (Human)) | BDBM50020476
(CHEMBL3290148)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C(=O)Nc1cnc(Nc2cccc(NC(=O)CNC(=O)C=C)c2)nc1 Show InChI InChI=1S/C31H26F3N7O4/c1-3-26(42)35-17-27(43)38-21-8-5-9-22(13-21)41-30-36-15-24(16-37-30)40-29(45)25-14-23(11-10-18(25)2)39-28(44)19-6-4-7-20(12-19)31(32,33)34/h3-16H,1,17H2,2H3,(H,35,42)(H,38,43)(H,39,44)(H,40,45)(H,36,37,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of Blk (unknown origin) after 1 hr by HTRF assay |
J Med Chem 57: 5112-28 (2014)
Article DOI: 10.1021/jm4017762
BindingDB Entry DOI: 10.7270/Q2NK3GKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
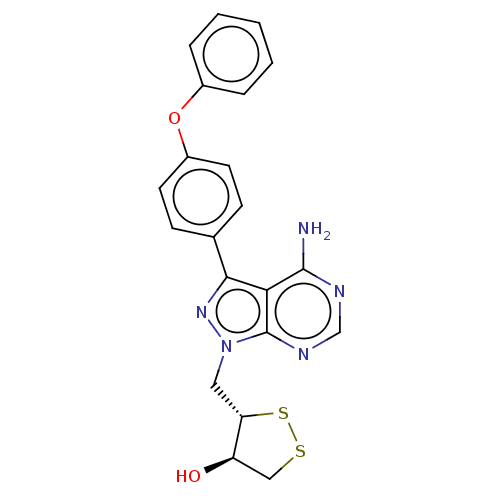
(Homo sapiens (Human)) | BDBM473174
((3S,4R)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@@H]3SSC[C@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50 Table 13-20: The protocol calls for test compound of the invention to be incubated with kinase, substrate, cofactors, and radio-isotope-labeled ... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
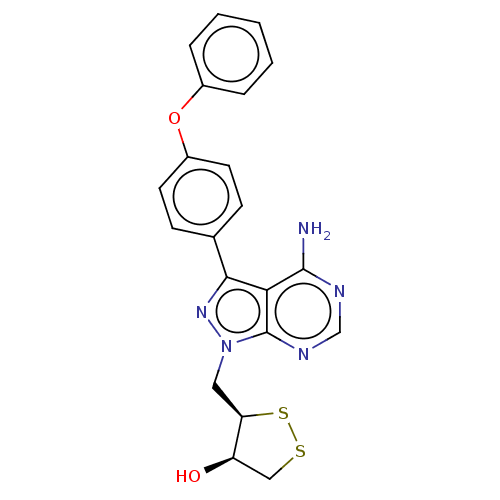
(Homo sapiens (Human)) | BDBM473172
((3R,4R)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@H]3SSC[C@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50 Table 13-20: The protocol calls for test compound of the invention to be incubated with kinase, substrate, cofactors, and radio-isotope-labeled ... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM230107
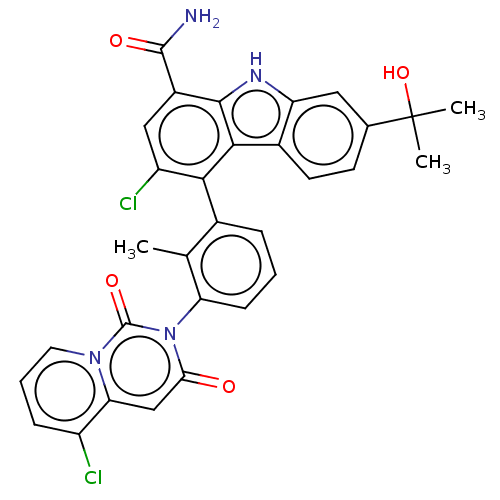
(US10106559, Example 33 | US10435415, Example 33 | ...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(Cl)cccn2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(3.03,8.9,;1.7,8.13,;.37,8.9,;-.97,8.13,;-.97,6.59,;.37,5.82,;1.7,6.59,;3.03,5.82,;3.03,4.28,;1.7,3.51,;4.37,3.51,;5.7,4.28,;7.03,3.51,;7.03,1.97,;8.37,4.28,;8.37,5.82,;7.03,6.59,;5.7,5.82,;4.37,6.59,;4.37,8.13,;.37,10.44,;1.7,11.21,;3.03,10.44,;1.7,12.75,;.37,13.52,;.37,15.06,;1.7,15.83,;-.97,15.83,;-.97,12.75,;-2.43,13.22,;-3.34,11.98,;-4.87,11.82,;-5.49,10.41,;-4.59,9.16,;-3.06,9.32,;-2.43,10.73,;-.97,11.21,;-7.03,10.41,;-6.64,8.92,;-8.37,9.64,;-7.8,11.74,)| Show InChI InChI=1S/C31H24Cl2N4O4/c1-15-17(6-4-8-23(15)37-25(38)14-24-20(32)7-5-11-36(24)30(37)40)26-21(33)13-19(29(34)39)28-27(26)18-10-9-16(31(2,3)41)12-22(18)35-28/h4-14,35,41H,1-3H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BLK (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM452966

(US10711006, Compound 15b)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccc(F)cc4)nc3)c12)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C24H22FN7O2/c1-2-20(33)31-11-3-4-17(13-31)32-24-21(23(26)28-14-29-24)22(30-32)15-5-10-19(27-12-15)34-18-8-6-16(25)7-9-18/h2,5-10,12,14,17H,1,3-4,11,13H2,(H2,26,28,29)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
HANGZHOU HERTZ PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Preparation of Compounds to be Tested:1) Using DMSO to prepare 50× compound stock solutions (same as the stock solution in Example 34) for later use;... |
US Patent US10711006 (2020)
BindingDB Entry DOI: 10.7270/Q2WH2T10 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50602801
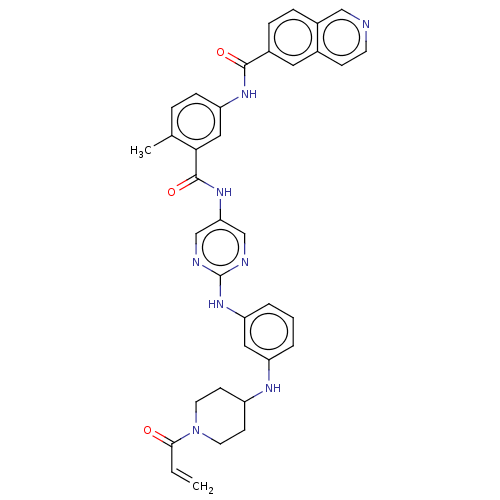
(CHEMBL5202420)Show SMILES Cc1ccc(NC(=O)c2ccc3cnccc3c2)cc1C(=O)Nc1cnc(Nc2cccc(NC3CCN(CC3)C(=O)C=C)c2)nc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114051
BindingDB Entry DOI: 10.7270/Q2HH6Q5N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM31096
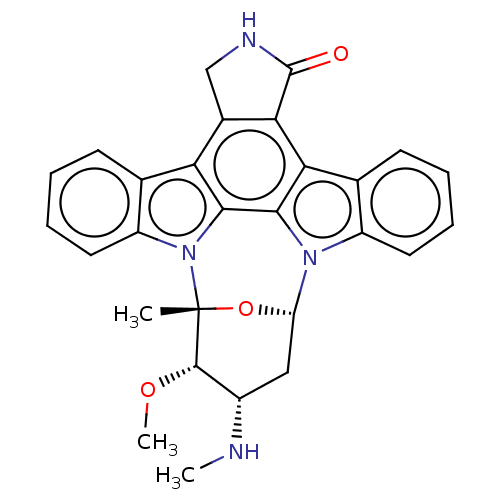
(CHEMBL290084 | Staurosporine | cid_451705)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | 25 |
East China University of Science and Technology
US Patent
| Assay Description
In vitro enzyme activity assay: wild-type and various mutants (T790M, L858R, L861Q, L858 R/T790M) EGFR, Z′-Lyte Kinase Assay Kit were purchased... |
US Patent US9670213 (2017)
BindingDB Entry DOI: 10.7270/Q23R0R1P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50602803
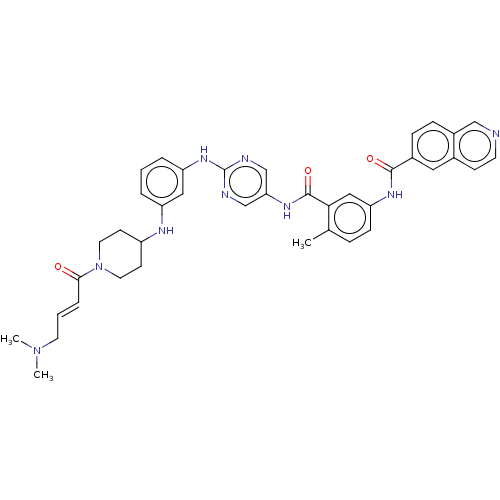
(CHEMBL5169523)Show SMILES CN(C)C\C=C\C(=O)N1CCC(CC1)Nc1cccc(Nc2ncc(NC(=O)c3cc(NC(=O)c4ccc5cnccc5c4)ccc3C)cn2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114051
BindingDB Entry DOI: 10.7270/Q2HH6Q5N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data