

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50614424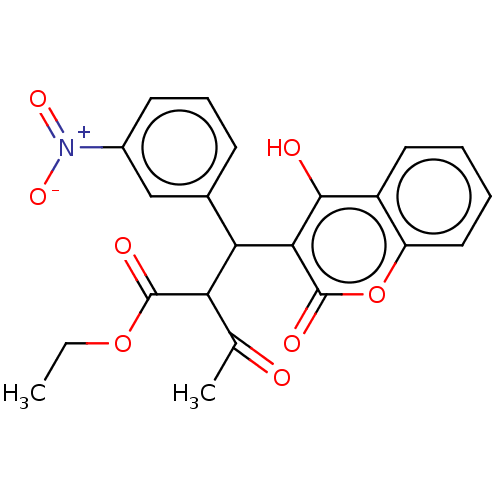 (CHEMBL478728) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037527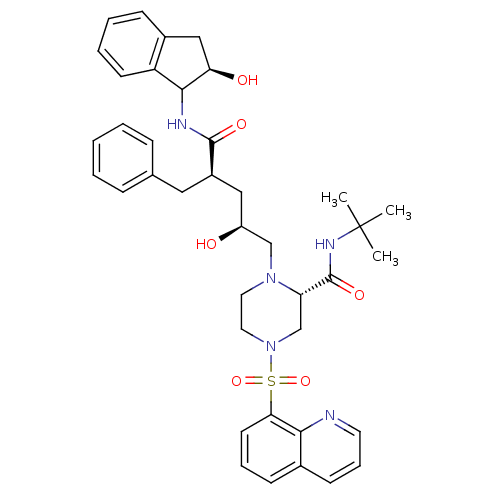 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the inhibition of HIV protease | J Med Chem 37: 3443-51 (1994) BindingDB Entry DOI: 10.7270/Q24J0D5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283729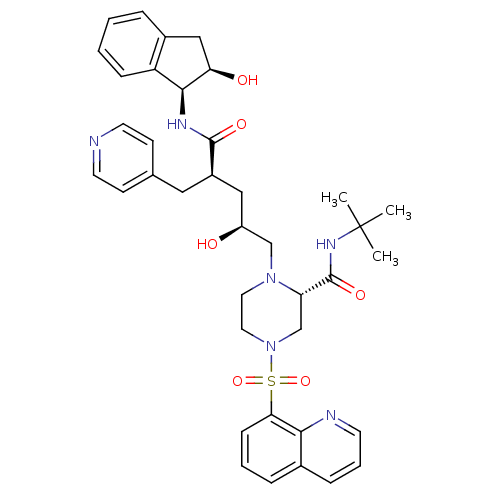 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM168608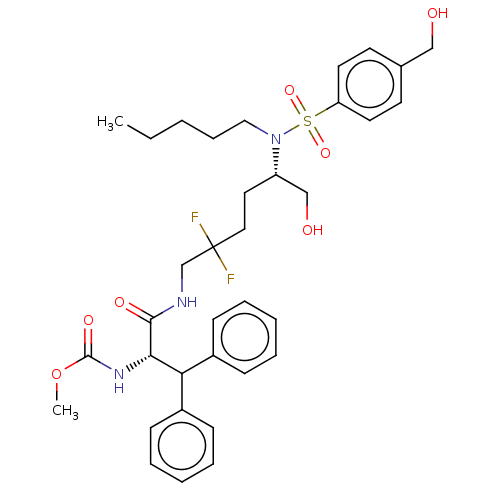 (US9079834, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Canada Inc. US Patent | Assay Description Assays for the inhibition of acute HIV infection of T-lymphoid cells were conducted in accordance with Vacca, J.P. et al, Proc. Natl. Acad. Sci. USA ... | US Patent US9079834 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50600465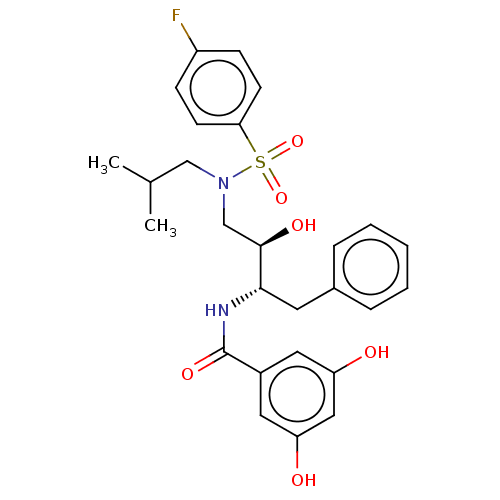 (CHEMBL5176682) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116760 BindingDB Entry DOI: 10.7270/Q29S1W3T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50562071 (CHEMBL319408) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00530 BindingDB Entry DOI: 10.7270/Q2W95DW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969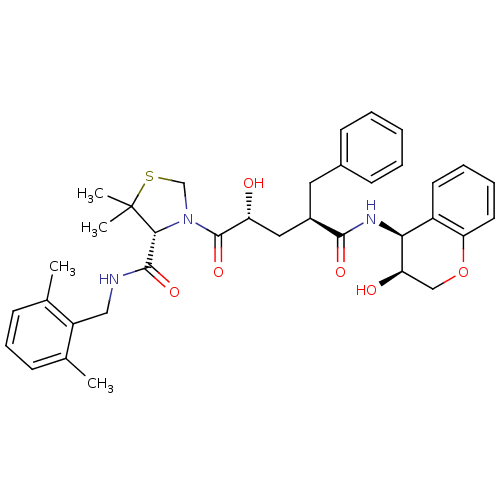 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM60467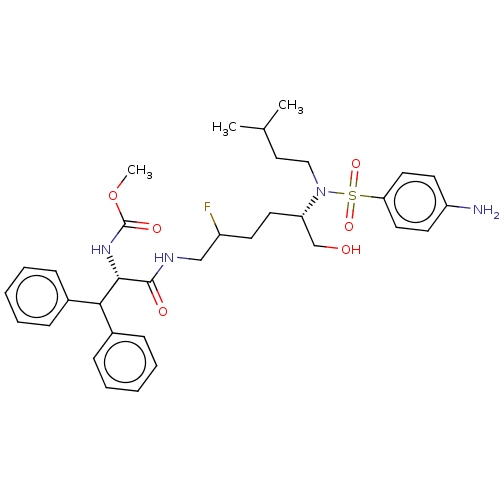 (BDBM168607 | US9079834, 1-1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Canada Inc. US Patent | Assay Description Assays for the inhibition of acute HIV infection of T-lymphoid cells were conducted in accordance with Vacca, J.P. et al, Proc. Natl. Acad. Sci. USA ... | US Patent US9079834 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283732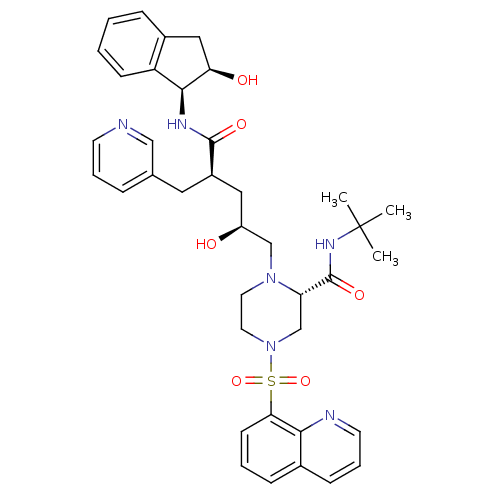 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50453057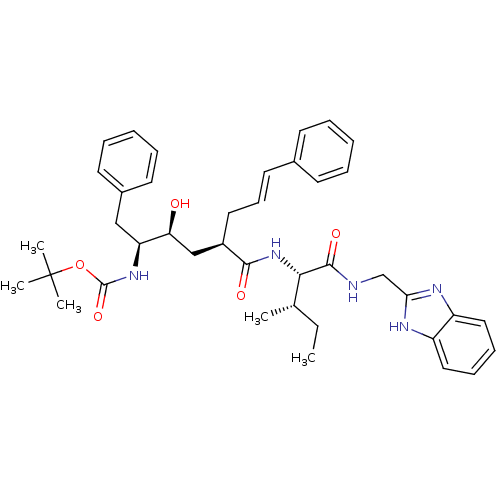 (CHEMBL3085527) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its affinity against HIV-1 protease | J Med Chem 34: 2305-14 (1991) BindingDB Entry DOI: 10.7270/Q2862H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383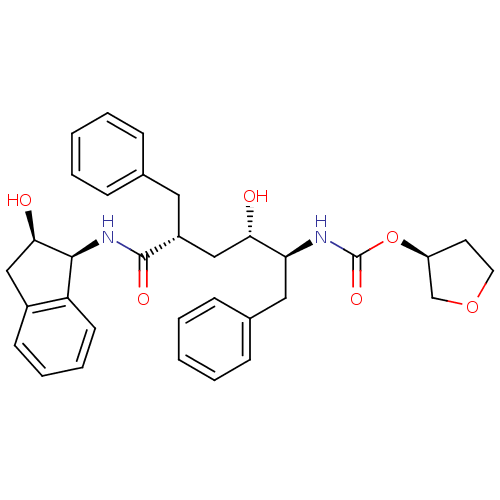 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 40: 2440-4 (1997) Article DOI: 10.1021/jm970195u BindingDB Entry DOI: 10.7270/Q2WD417W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283726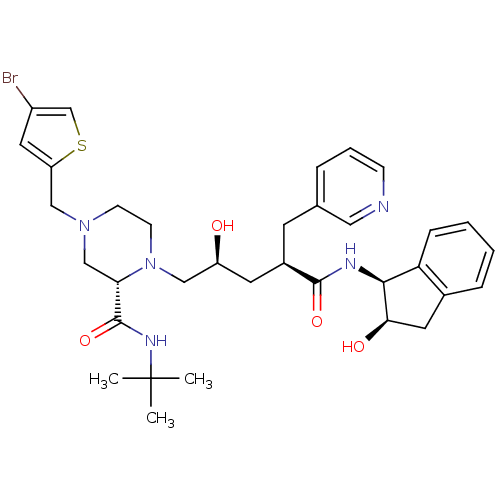 ((S)-4-(4-Bromo-thiophen-2-ylmethyl)-1-[(2S,4R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970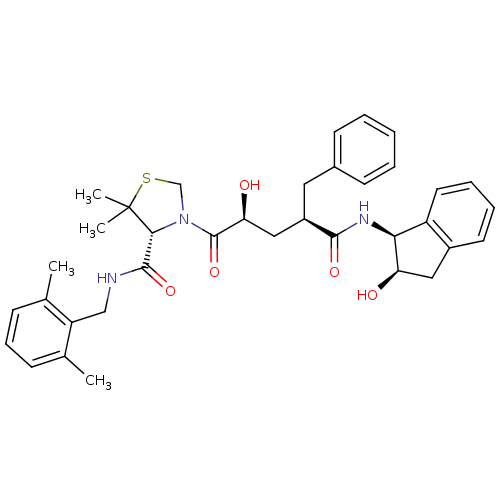 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285450 (CHEMBL300140 | [(1S,2R)-1-Benzyl-3-((S)-4-[2,2']bi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV-1 Protease | Bioorg Med Chem Lett 5: 185-190 (1995) Article DOI: 10.1016/0960-894X(95)00005-E BindingDB Entry DOI: 10.7270/Q2JS9QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127976 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9291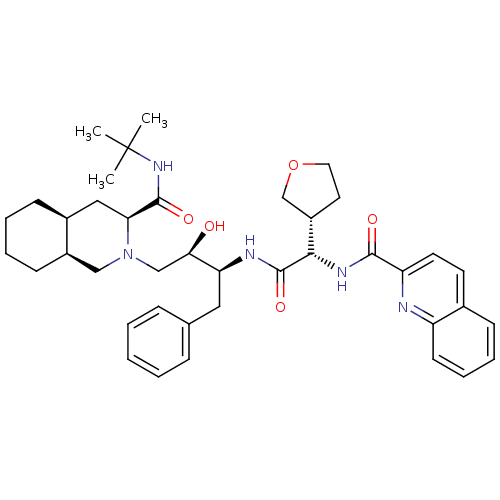 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease was determined in vitro | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283723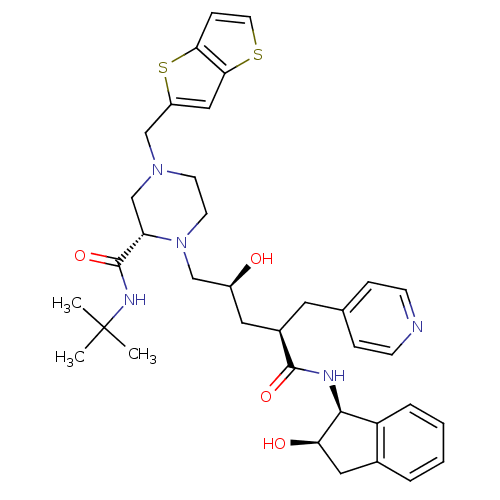 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9291 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£ Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease. | J Med Chem 41: 836-52 (1998) Article DOI: 10.1021/jm970535b BindingDB Entry DOI: 10.7270/Q21R6RTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1277 (L-682, 679 analog 36 | tert-butyl N-[(2S,3S,5R)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Sharp and Dohme Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 2852-7 (1991) Article DOI: 10.1021/jm00113a025 BindingDB Entry DOI: 10.7270/Q2M906TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127977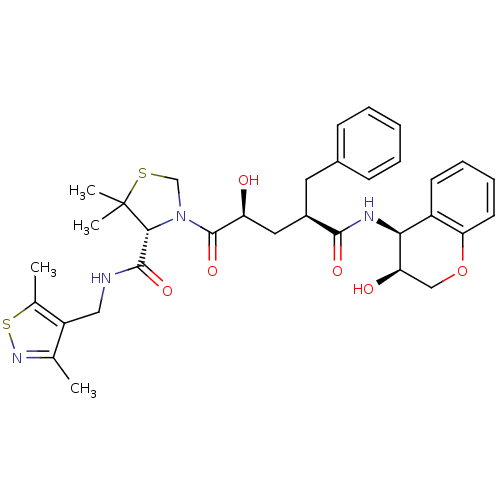 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50550778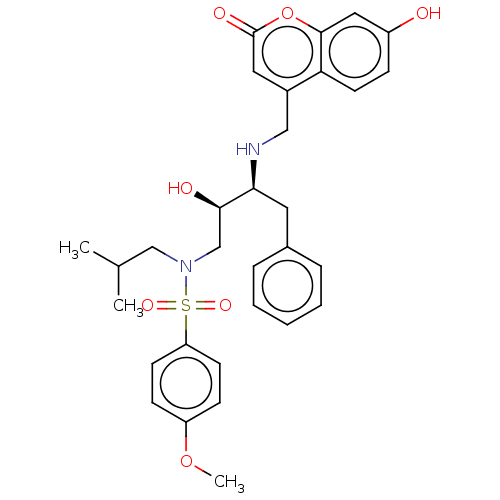 (CHEMBL4741886) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant HIV1 protease measured assessed as hydrolysis of fluorogenic substrate by FRET assay | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111900 BindingDB Entry DOI: 10.7270/Q2D79G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50562070 (CHEMBL17612) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease using H-Val-Ser-Gln-Am-(L-b-naphthyl-alanine)-Pro-Ile-Val-OH as substrate by HPLC method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00530 BindingDB Entry DOI: 10.7270/Q2W95DW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against Human immunodeficiency virus (HIV-1) protease | Bioorg Med Chem Lett 4: 499-504 (1994) Article DOI: 10.1016/0960-894X(94)80025-1 BindingDB Entry DOI: 10.7270/Q2GM8777 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£ Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease. | J Med Chem 41: 836-52 (1998) Article DOI: 10.1021/jm970535b BindingDB Entry DOI: 10.7270/Q21R6RTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50422338 (CHEMBL136146) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibition of human immunodeficiency virus type 1 (HIV-1) protease enzyme. | J Med Chem 43: 4446-51 (2000) BindingDB Entry DOI: 10.7270/Q2S75HMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease was determined in vitro | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037530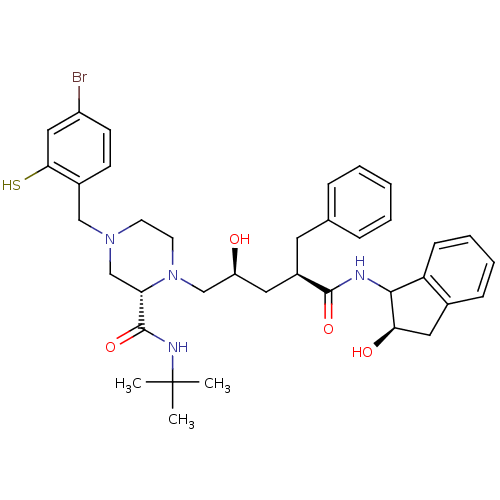 ((S)-4-(4-Bromo-2-mercapto-benzyl)-1-[(2S,4R)-2-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the inhibition of HIV protease | J Med Chem 37: 3443-51 (1994) BindingDB Entry DOI: 10.7270/Q24J0D5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283722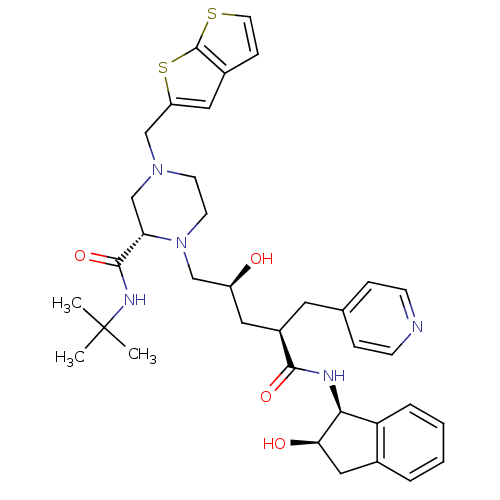 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50600447 (CHEMBL5174561) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116760 BindingDB Entry DOI: 10.7270/Q29S1W3T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9141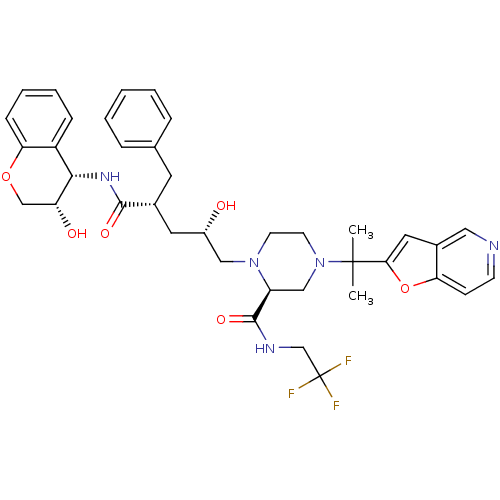 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against wild-type HIV-1 protease | Bioorg Med Chem Lett 12: 2423-6 (2002) BindingDB Entry DOI: 10.7270/Q2K35T0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127968 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127972 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50116913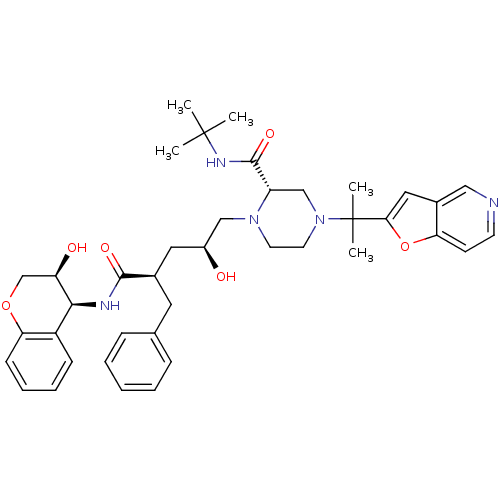 ((S)-4-(1-Furo[3,2-c]pyridin-2-yl-1-methyl-ethyl)-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit cleavage of a substrate by the wild-type HIV-1 protease enzyme | Bioorg Med Chem Lett 12: 2419-22 (2002) BindingDB Entry DOI: 10.7270/Q2PV6JQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50600463 (CHEMBL5200970) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116760 BindingDB Entry DOI: 10.7270/Q29S1W3T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50600454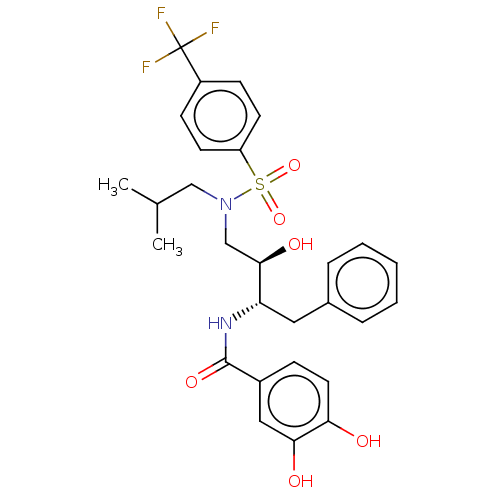 (CHEMBL5204718) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116760 BindingDB Entry DOI: 10.7270/Q29S1W3T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50600456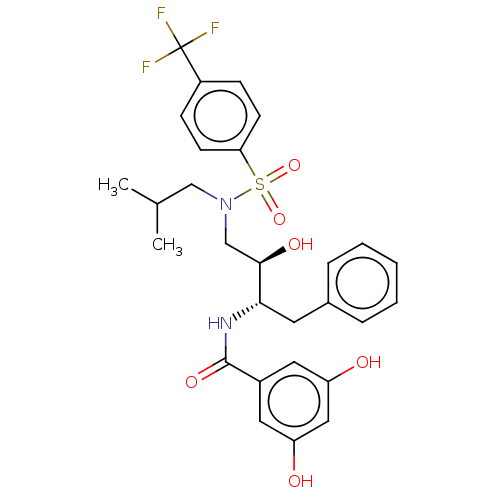 (CHEMBL5193294) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116760 BindingDB Entry DOI: 10.7270/Q29S1W3T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50116913 ((S)-4-(1-Furo[3,2-c]pyridin-2-yl-1-methyl-ethyl)-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against wild-type HIV-1 protease | Bioorg Med Chem Lett 12: 2423-6 (2002) BindingDB Entry DOI: 10.7270/Q2K35T0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50572548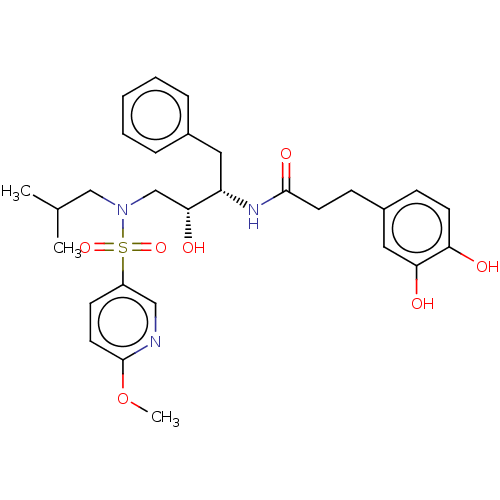 (CHEMBL4865743) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113498 BindingDB Entry DOI: 10.7270/Q2XW4PMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50604234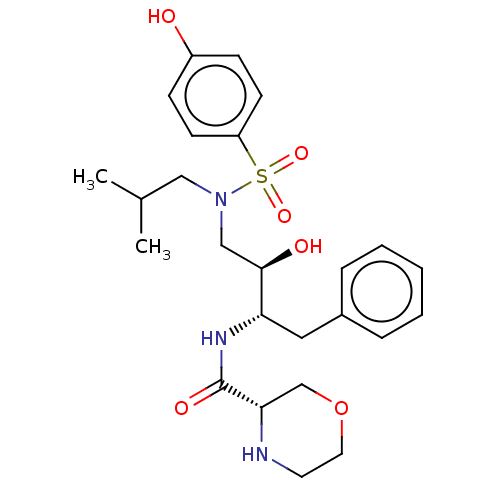 (CHEMBL5179241) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114251 BindingDB Entry DOI: 10.7270/Q2222ZWX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180212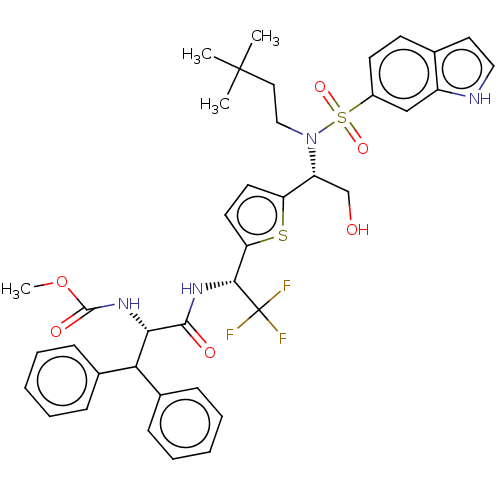 (US9133157, 94) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM60623 (BDBM180165 | US9133157, 103) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283733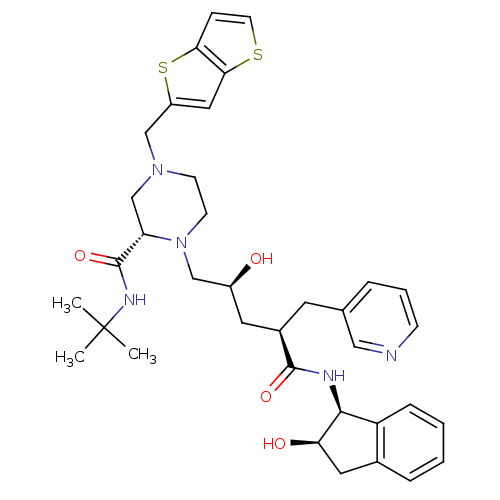 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM842 (Benzocycloalkyl Amines deriv. 12 | CHEMBL419923 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£ Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease. | J Med Chem 41: 836-52 (1998) Article DOI: 10.1021/jm970535b BindingDB Entry DOI: 10.7270/Q21R6RTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50422319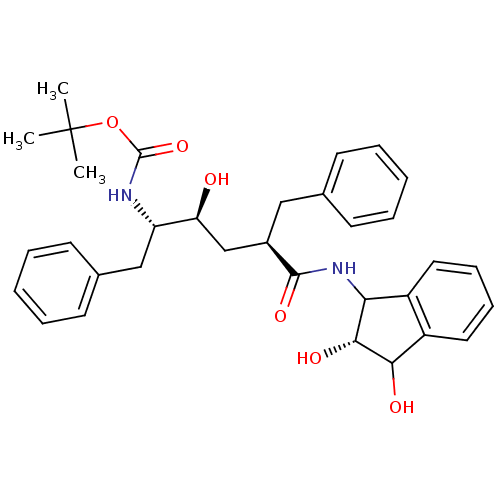 (CHEMBL138828) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibition of human immunodeficiency virus type 1 (HIV-1) protease enzyme. | J Med Chem 43: 4446-51 (2000) BindingDB Entry DOI: 10.7270/Q2S75HMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50118961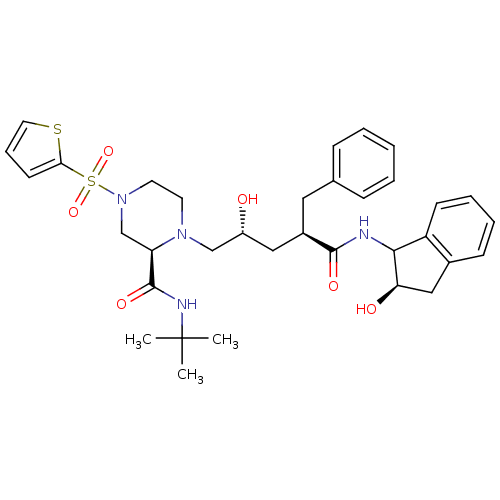 ((R)-1-[(3R,5R)-2-Hydroxy-4-((R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to prevent cleavage of acompound by the HIV protease wild-type enzyme | Bioorg Med Chem Lett 12: 2855-8 (2002) BindingDB Entry DOI: 10.7270/Q2TB167H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5297 total ) | Next | Last >> |