 Found 80 hits of ec50 for UniProtKB: P37230
Found 80 hits of ec50 for UniProtKB: P37230 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227669

((R)-2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CCN([C@@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227674
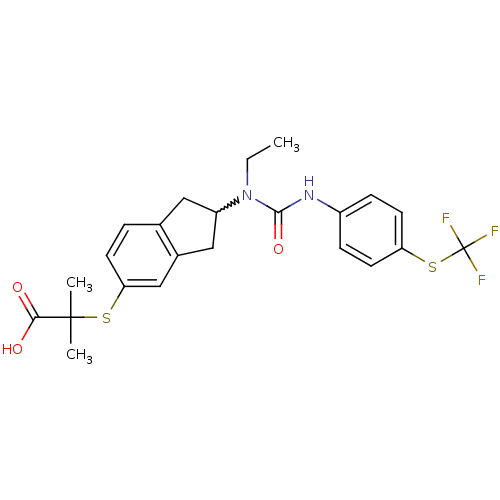
(2-(2-(1-ethyl-3-(4-(trifluoromethylthio)phenyl)ure...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:3.2| Show InChI InChI=1S/C23H25F3N2O3S2/c1-4-28(21(31)27-16-6-9-18(10-7-16)33-23(24,25)26)17-11-14-5-8-19(13-15(14)12-17)32-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227656
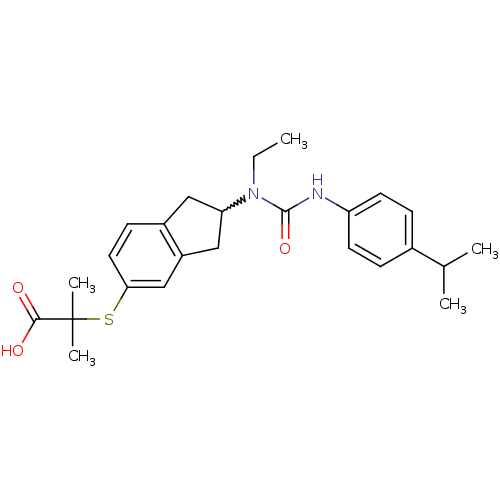
(2-(2-(1-ethyl-3-(4-isopropylphenyl)ureido)-2,3-dih...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:3.2| Show InChI InChI=1S/C25H32N2O3S/c1-6-27(24(30)26-20-10-7-17(8-11-20)16(2)3)21-13-18-9-12-22(15-19(18)14-21)31-25(4,5)23(28)29/h7-12,15-16,21H,6,13-14H2,1-5H3,(H,26,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227664

(2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ureido...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:3.2| Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227704

(2-methyl-2-(2-(1-pentyl-3-(4-(trifluoromethylthio)...)Show SMILES CCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:6.5| Show InChI InChI=1S/C26H31F3N2O3S2/c1-4-5-6-13-31(24(34)30-19-8-11-21(12-9-19)36-26(27,28)29)20-14-17-7-10-22(16-18(17)15-20)35-25(2,3)23(32)33/h7-12,16,20H,4-6,13-15H2,1-3H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227696
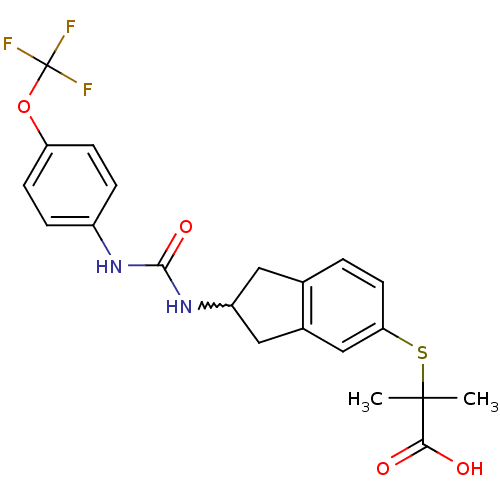
(2-methyl-2-(2-(3-(4-(trifluoromethoxy)phenyl)ureid...)Show SMILES CC(C)(Sc1ccc2CC(Cc2c1)NC(=O)Nc1ccc(OC(F)(F)F)cc1)C(O)=O |w:9.14| Show InChI InChI=1S/C21H21F3N2O4S/c1-20(2,18(27)28)31-17-8-3-12-9-15(10-13(12)11-17)26-19(29)25-14-4-6-16(7-5-14)30-21(22,23)24/h3-8,11,15H,9-10H2,1-2H3,(H,27,28)(H2,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 96 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227663
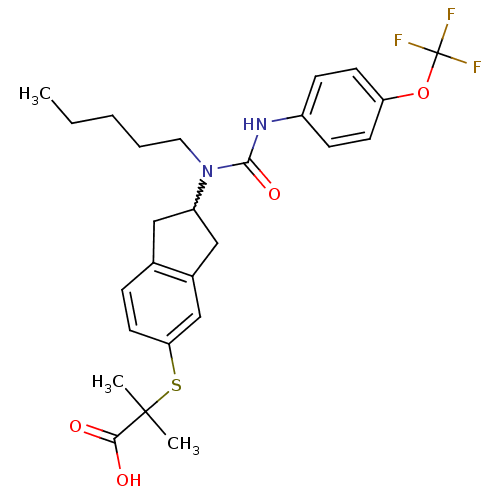
(2-methyl-2-(2-(1-pentyl-3-(4-(trifluoromethoxy)phe...)Show SMILES CCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:6.5| Show InChI InChI=1S/C26H31F3N2O4S/c1-4-5-6-13-31(24(34)30-19-8-10-21(11-9-19)35-26(27,28)29)20-14-17-7-12-22(16-18(17)15-20)36-25(2,3)23(32)33/h7-12,16,20H,4-6,13-15H2,1-3H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 105 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227677
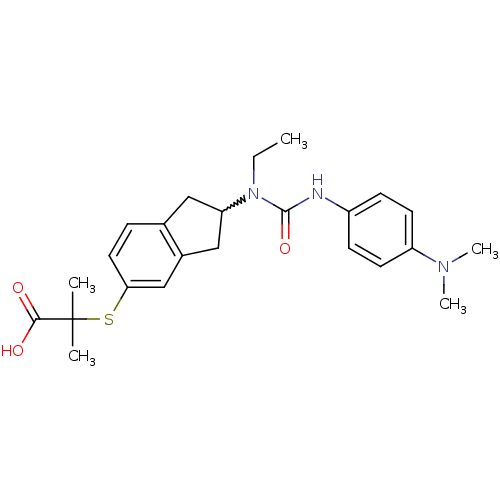
(2-(2-(3-(4-(dimethylamino)phenyl)-1-ethylureido)-2...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:3.2| Show InChI InChI=1S/C24H31N3O3S/c1-6-27(23(30)25-18-8-10-19(11-9-18)26(4)5)20-13-16-7-12-21(15-17(16)14-20)31-24(2,3)22(28)29/h7-12,15,20H,6,13-14H2,1-5H3,(H,25,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM28802

(2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...)Show SMILES COC(=O)N(CC(O)=O)Cc1cc(OCc2nc(oc2C)-c2ccc(Cl)cc2)ccc1F Show InChI InChI=1S/C22H20ClFN2O6/c1-13-19(25-21(32-13)14-3-5-16(23)6-4-14)12-31-17-7-8-18(24)15(9-17)10-26(11-20(27)28)22(29)30-2/h3-9H,10-12H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... |
J Pharmacol Exp Ther 327: 716-26 (2008)
Article DOI: 10.1124/jpet.108.143271
BindingDB Entry DOI: 10.7270/Q2VD6WT9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM28802

(2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...)Show SMILES COC(=O)N(CC(O)=O)Cc1cc(OCc2nc(oc2C)-c2ccc(Cl)cc2)ccc1F Show InChI InChI=1S/C22H20ClFN2O6/c1-13-19(25-21(32-13)14-3-5-16(23)6-4-14)12-31-17-7-8-18(24)15(9-17)10-26(11-20(27)28)22(29)30-2/h3-9H,10-12H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPAR-alpha |
ACS Med Chem Lett 7: 590-4 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00033
BindingDB Entry DOI: 10.7270/Q2CR5W99 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227658
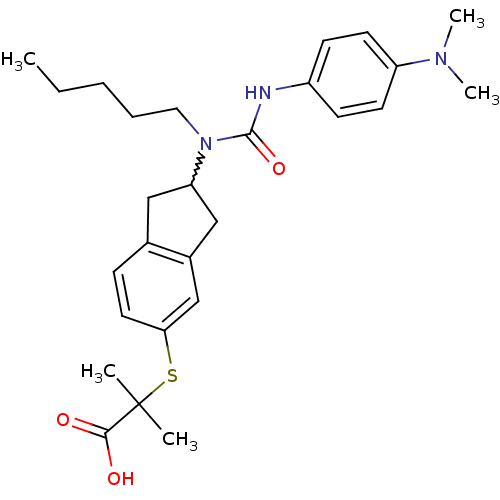
(2-(2-(3-(4-(dimethylamino)phenyl)-1-pentylureido)-...)Show SMILES CCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:6.5| Show InChI InChI=1S/C27H37N3O3S/c1-6-7-8-15-30(26(33)28-21-10-12-22(13-11-21)29(4)5)23-16-19-9-14-24(18-20(19)17-23)34-27(2,3)25(31)32/h9-14,18,23H,6-8,15-17H2,1-5H3,(H,28,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227660
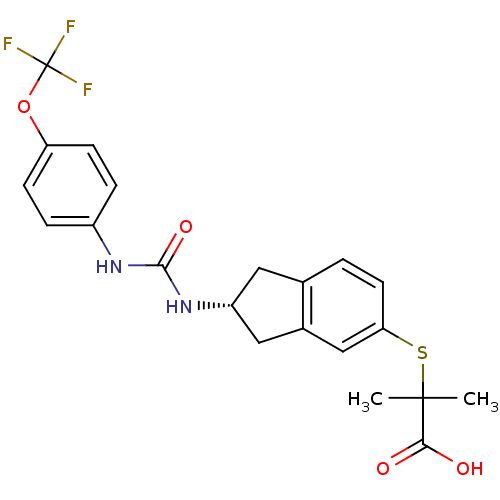
((R)-2-methyl-2-(2-(3-(4-(trifluoromethoxy)phenyl)u...)Show SMILES CC(C)(Sc1ccc2C[C@H](Cc2c1)NC(=O)Nc1ccc(OC(F)(F)F)cc1)C(O)=O Show InChI InChI=1S/C21H21F3N2O4S/c1-20(2,18(27)28)31-17-8-3-12-9-15(10-13(12)11-17)26-19(29)25-14-4-6-16(7-5-14)30-21(22,23)24/h3-8,11,15H,9-10H2,1-2H3,(H,27,28)(H2,25,26,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 146 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227668
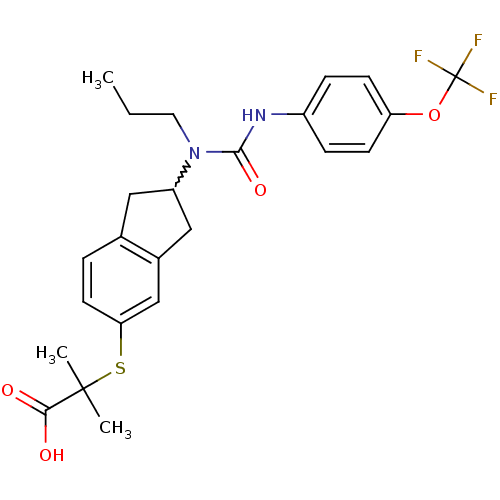
(2-methyl-2-(2-(1-propyl-3-(4-(trifluoromethoxy)phe...)Show SMILES CCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:4.3| Show InChI InChI=1S/C24H27F3N2O4S/c1-4-11-29(22(32)28-17-6-8-19(9-7-17)33-24(25,26)27)18-12-15-5-10-20(14-16(15)13-18)34-23(2,3)21(30)31/h5-10,14,18H,4,11-13H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227659
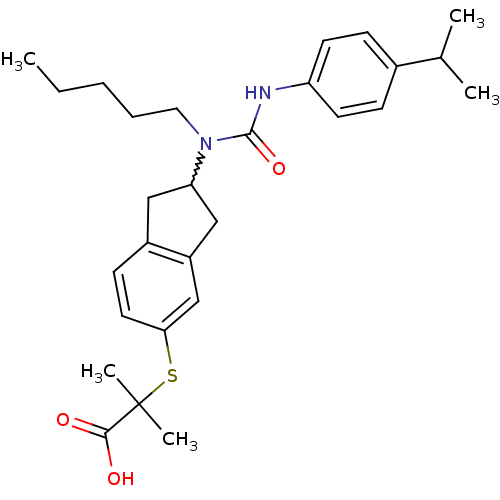
(2-(2-(3-(4-isopropylphenyl)-1-pentylureido)-2,3-di...)Show SMILES CCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:6.5| Show InChI InChI=1S/C28H38N2O3S/c1-6-7-8-15-30(27(33)29-23-12-9-20(10-13-23)19(2)3)24-16-21-11-14-25(18-22(21)17-24)34-28(4,5)26(31)32/h9-14,18-19,24H,6-8,15-17H2,1-5H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 159 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227666
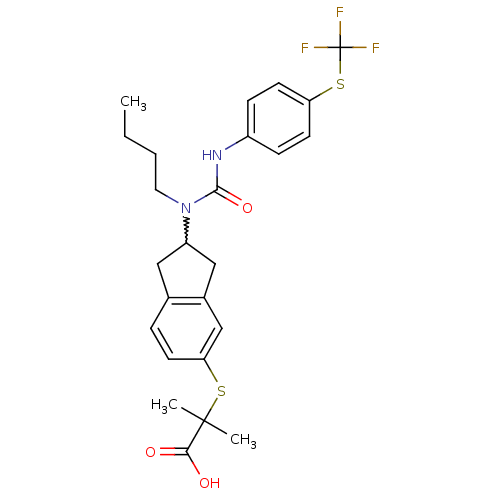
(2-(2-(1-butyl-3-(4-(trifluoromethylthio)phenyl)ure...)Show SMILES CCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C25H29F3N2O3S2/c1-4-5-12-30(23(33)29-18-7-10-20(11-8-18)35-25(26,27)28)19-13-16-6-9-21(15-17(16)14-19)34-24(2,3)22(31)32/h6-11,15,19H,4-5,12-14H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227675
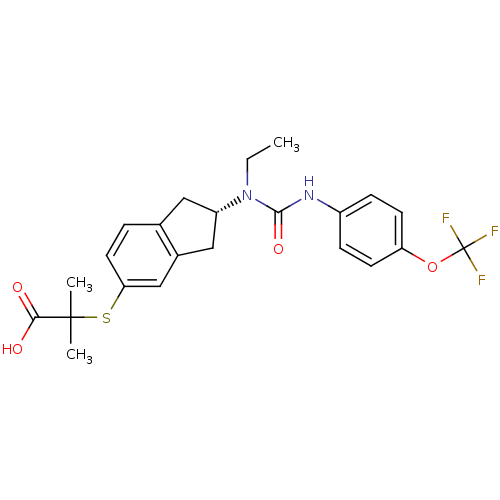
((S)-2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CCN([C@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227688
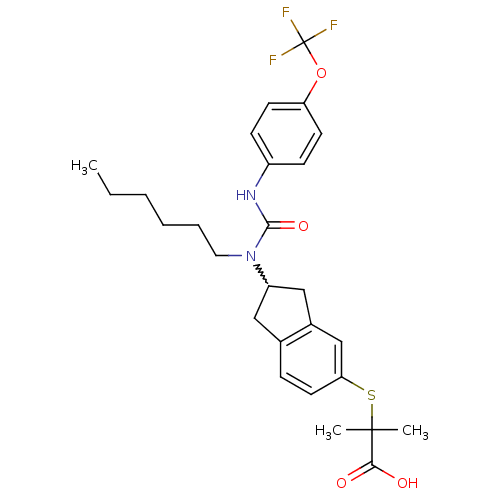
(2-(2-(1-hexyl-3-(4-(trifluoromethoxy)phenyl)ureido...)Show SMILES CCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:7.6| Show InChI InChI=1S/C27H33F3N2O4S/c1-4-5-6-7-14-32(25(35)31-20-9-11-22(12-10-20)36-27(28,29)30)21-15-18-8-13-23(17-19(18)16-21)37-26(2,3)24(33)34/h8-13,17,21H,4-7,14-16H2,1-3H3,(H,31,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM28799
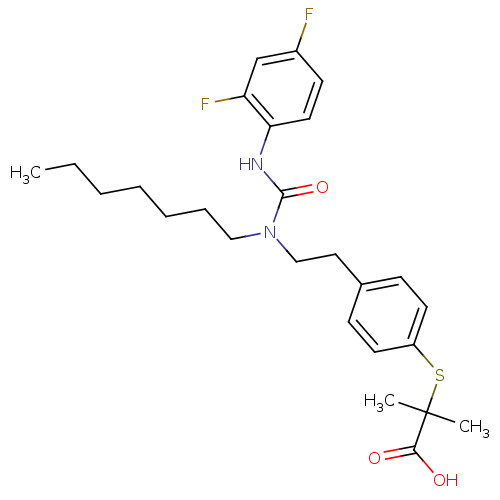
(2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)a...)Show SMILES CCCCCCCN(CCc1ccc(SC(C)(C)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H34F2N2O3S/c1-4-5-6-7-8-16-30(25(33)29-23-14-11-20(27)18-22(23)28)17-15-19-9-12-21(13-10-19)34-26(2,3)24(31)32/h9-14,18H,4-8,15-17H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227705
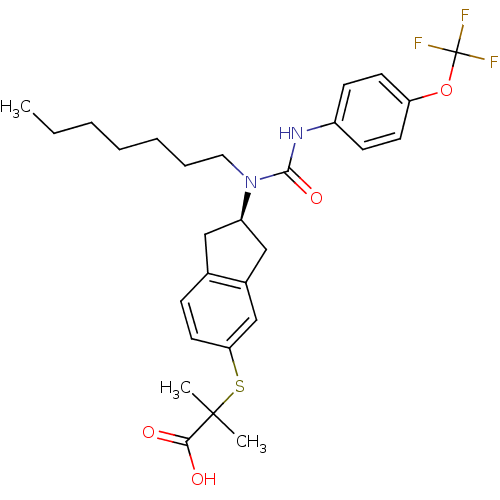
((R)-2-(2-(1-heptyl-3-(4-(trifluoromethoxy)phenyl)u...)Show SMILES CCCCCCCN([C@@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H35F3N2O4S/c1-4-5-6-7-8-15-33(26(36)32-21-10-12-23(13-11-21)37-28(29,30)31)22-16-19-9-14-24(18-20(19)17-22)38-27(2,3)25(34)35/h9-14,18,22H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 169 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227678
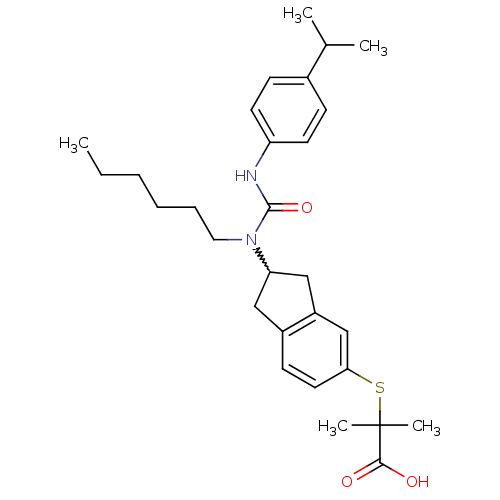
(2-(2-(1-hexyl-3-(4-isopropylphenyl)ureido)-2,3-dih...)Show SMILES CCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:7.6| Show InChI InChI=1S/C29H40N2O3S/c1-6-7-8-9-16-31(28(34)30-24-13-10-21(11-14-24)20(2)3)25-17-22-12-15-26(19-23(22)18-25)35-29(4,5)27(32)33/h10-15,19-20,25H,6-9,16-18H2,1-5H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227661
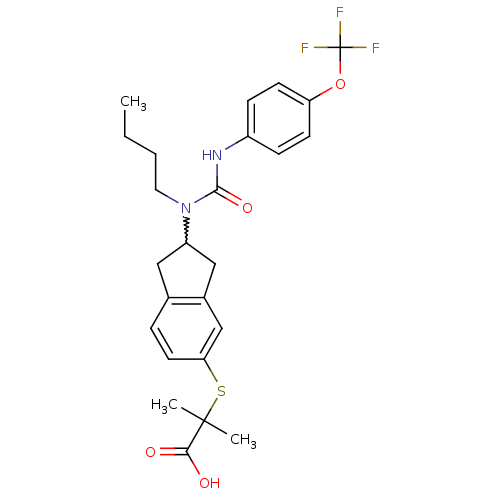
(2-(2-(1-butyl-3-(4-(trifluoromethoxy)phenyl)ureido...)Show SMILES CCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C25H29F3N2O4S/c1-4-5-12-30(23(33)29-18-7-9-20(10-8-18)34-25(26,27)28)19-13-16-6-11-21(15-17(16)14-19)35-24(2,3)22(31)32/h6-11,15,19H,4-5,12-14H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227657
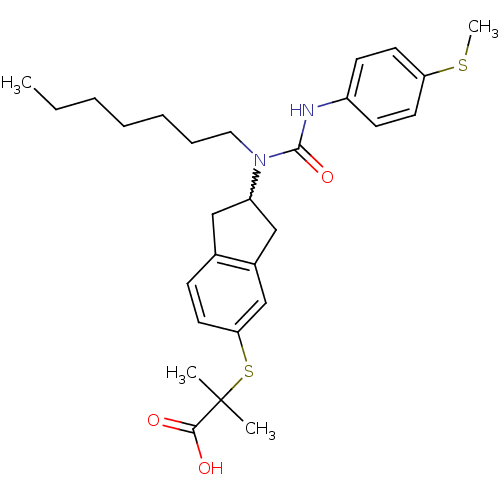
(2-(2-(1-heptyl-3-(4-(methylthio)phenyl)ureido)-2,3...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC)cc1 |w:8.7| Show InChI InChI=1S/C28H38N2O3S2/c1-5-6-7-8-9-16-30(27(33)29-22-11-14-24(34-4)15-12-22)23-17-20-10-13-25(19-21(20)18-23)35-28(2,3)26(31)32/h10-15,19,23H,5-9,16-18H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227682
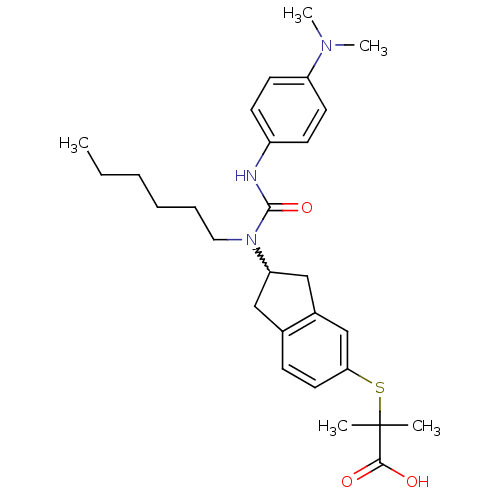
(2-(2-(3-(4-(dimethylamino)phenyl)-1-hexylureido)-2...)Show SMILES CCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:7.6| Show InChI InChI=1S/C28H39N3O3S/c1-6-7-8-9-16-31(27(34)29-22-11-13-23(14-12-22)30(4)5)24-17-20-10-15-25(19-21(20)18-24)35-28(2,3)26(32)33/h10-15,19,24H,6-9,16-18H2,1-5H3,(H,29,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 219 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227703
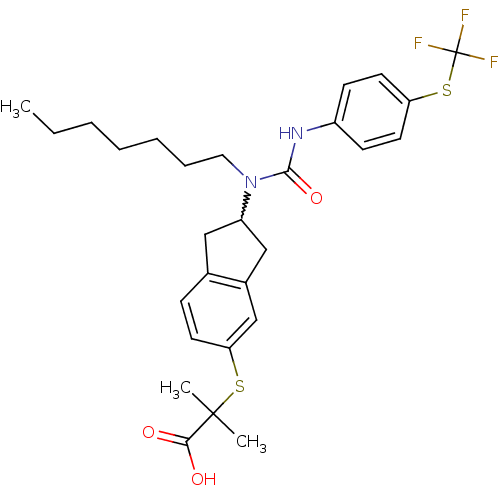
(2-(2-(1-heptyl-3-(4-(trifluoromethylthio)phenyl)ur...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S2/c1-4-5-6-7-8-15-33(26(36)32-21-10-13-23(14-11-21)38-28(29,30)31)22-16-19-9-12-24(18-20(19)17-22)37-27(2,3)25(34)35/h9-14,18,22H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 229 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227685
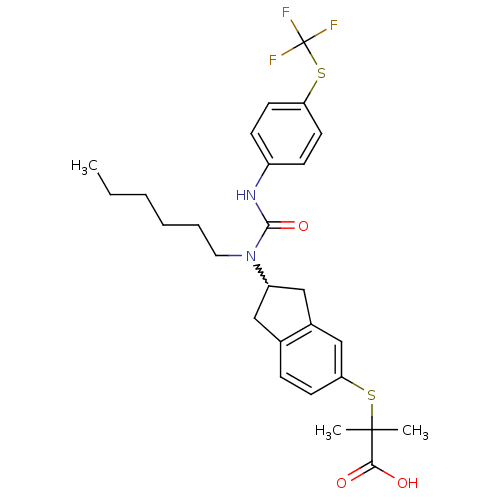
(2-(2-(1-hexyl-3-(4-(trifluoromethylthio)phenyl)ure...)Show SMILES CCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:7.6| Show InChI InChI=1S/C27H33F3N2O3S2/c1-4-5-6-7-14-32(25(35)31-20-9-12-22(13-10-20)37-27(28,29)30)21-15-18-8-11-23(17-19(18)16-21)36-26(2,3)24(33)34/h8-13,17,21H,4-7,14-16H2,1-3H3,(H,31,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 249 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227671
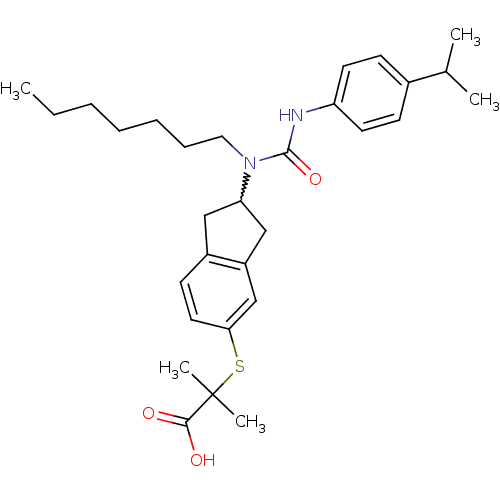
(2-(2-(1-heptyl-3-(4-isopropylphenyl)ureido)-2,3-di...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:8.7| Show InChI InChI=1S/C30H42N2O3S/c1-6-7-8-9-10-17-32(29(35)31-25-14-11-22(12-15-25)21(2)3)26-18-23-13-16-27(20-24(23)19-26)36-30(4,5)28(33)34/h11-16,20-21,26H,6-10,17-19H2,1-5H3,(H,31,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 252 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227662
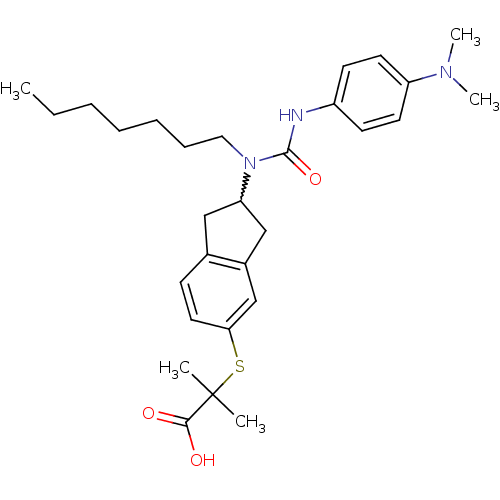
(2-(2-(3-(4-(dimethylamino)phenyl)-1-heptylureido)-...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:8.7| Show InChI InChI=1S/C29H41N3O3S/c1-6-7-8-9-10-17-32(28(35)30-23-12-14-24(15-13-23)31(4)5)25-18-21-11-16-26(20-22(21)19-25)36-29(2,3)27(33)34/h11-16,20,25H,6-10,17-19H2,1-5H3,(H,30,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 252 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227687
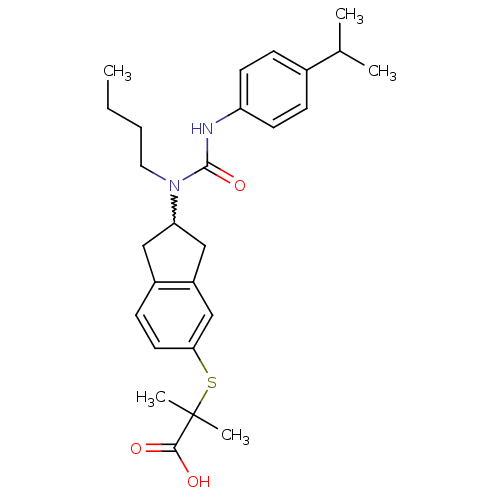
(2-(2-(1-butyl-3-(4-isopropylphenyl)ureido)-2,3-dih...)Show SMILES CCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:5.4| Show InChI InChI=1S/C27H36N2O3S/c1-6-7-14-29(26(32)28-22-11-8-19(9-12-22)18(2)3)23-15-20-10-13-24(17-21(20)16-23)33-27(4,5)25(30)31/h8-13,17-18,23H,6-7,14-16H2,1-5H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227683
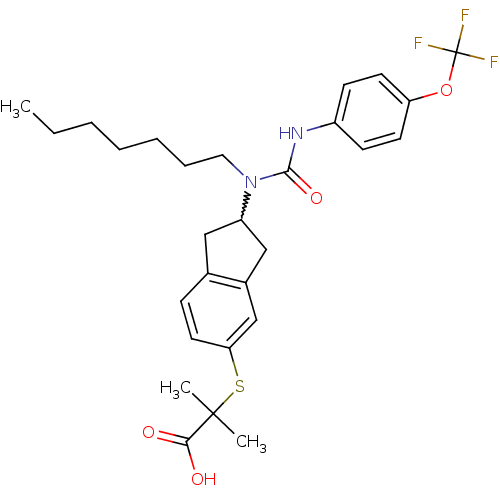
(2-(2-(1-heptyl-3-(4-(trifluoromethoxy)phenyl)ureid...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:8.7| Show InChI InChI=1S/C28H35F3N2O4S/c1-4-5-6-7-8-15-33(26(36)32-21-10-12-23(13-11-21)37-28(29,30)31)22-16-19-9-14-24(18-20(19)17-22)38-27(2,3)25(34)35/h9-14,18,22H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 294 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM28800
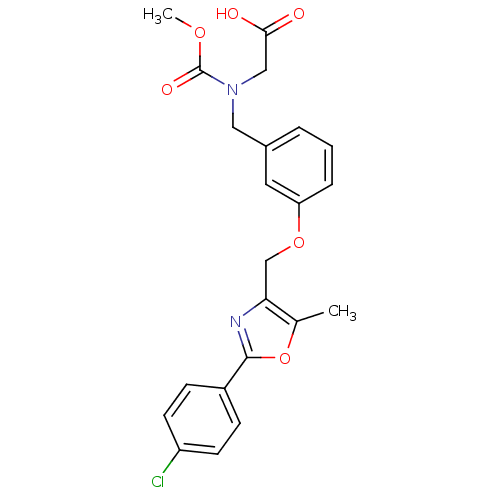
(2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...)Show SMILES COC(=O)N(CC(O)=O)Cc1cccc(OCc2nc(oc2C)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C22H21ClN2O6/c1-14-19(24-21(31-14)16-6-8-17(23)9-7-16)13-30-18-5-3-4-15(10-18)11-25(12-20(26)27)22(28)29-2/h3-10H,11-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 317 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... |
J Pharmacol Exp Ther 327: 716-26 (2008)
Article DOI: 10.1124/jpet.108.143271
BindingDB Entry DOI: 10.7270/Q2VD6WT9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM28800

(2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...)Show SMILES COC(=O)N(CC(O)=O)Cc1cccc(OCc2nc(oc2C)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C22H21ClN2O6/c1-14-19(24-21(31-14)16-6-8-17(23)9-7-16)13-30-18-5-3-4-15(10-18)11-25(12-20(26)27)22(28)29-2/h3-10H,11-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 317 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPAR-alpha |
ACS Med Chem Lett 7: 590-4 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00033
BindingDB Entry DOI: 10.7270/Q2CR5W99 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227692
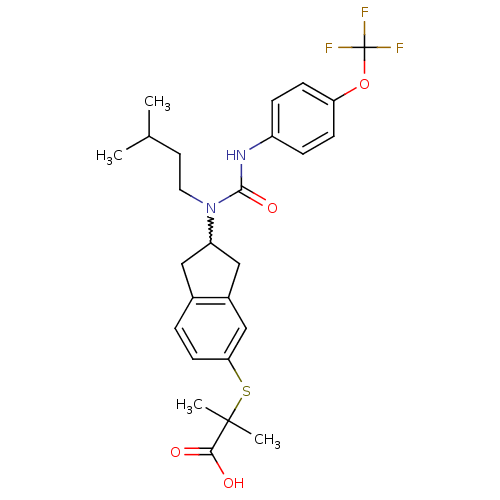
(2-(2-(1-isopentyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CC(C)CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:6.5| Show InChI InChI=1S/C26H31F3N2O4S/c1-16(2)11-12-31(24(34)30-19-6-8-21(9-7-19)35-26(27,28)29)20-13-17-5-10-22(15-18(17)14-20)36-25(3,4)23(32)33/h5-10,15-16,20H,11-14H2,1-4H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 386 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227690
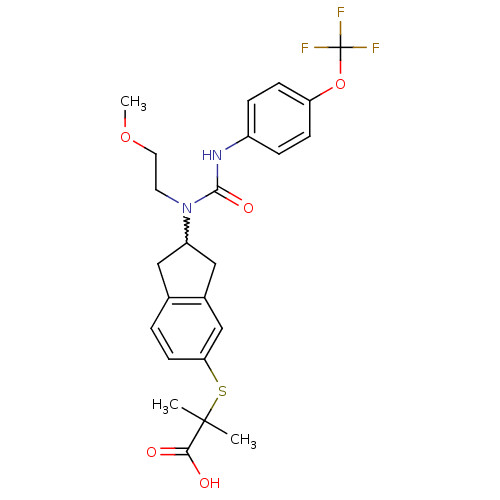
(2-(2-(1-(2-methoxyethyl)-3-(4-(trifluoromethoxy)ph...)Show SMILES COCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C24H27F3N2O5S/c1-23(2,21(30)31)35-20-9-4-15-12-18(13-16(15)14-20)29(10-11-33-3)22(32)28-17-5-7-19(8-6-17)34-24(25,26)27/h4-9,14,18H,10-13H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 425 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227679
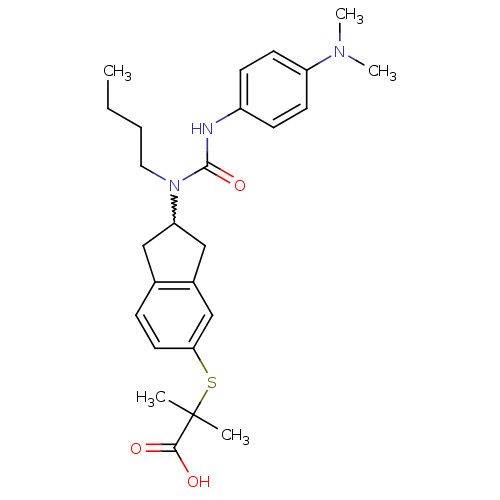
(2-(2-(1-butyl-3-(4-(dimethylamino)phenyl)ureido)-2...)Show SMILES CCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:5.4| Show InChI InChI=1S/C26H35N3O3S/c1-6-7-14-29(25(32)27-20-9-11-21(12-10-20)28(4)5)22-15-18-8-13-23(17-19(18)16-22)33-26(2,3)24(30)31/h8-13,17,22H,6-7,14-16H2,1-5H3,(H,27,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 561 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227697
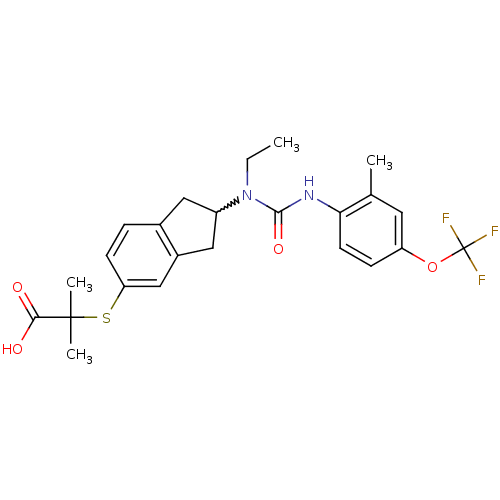
(2-(2-(1-ethyl-3-(2-methyl-4-(trifluoromethoxy)phen...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1C |w:3.2| Show InChI InChI=1S/C24H27F3N2O4S/c1-5-29(22(32)28-20-9-7-18(10-14(20)2)33-24(25,26)27)17-11-15-6-8-19(13-16(15)12-17)34-23(3,4)21(30)31/h6-10,13,17H,5,11-12H2,1-4H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 598 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50134840
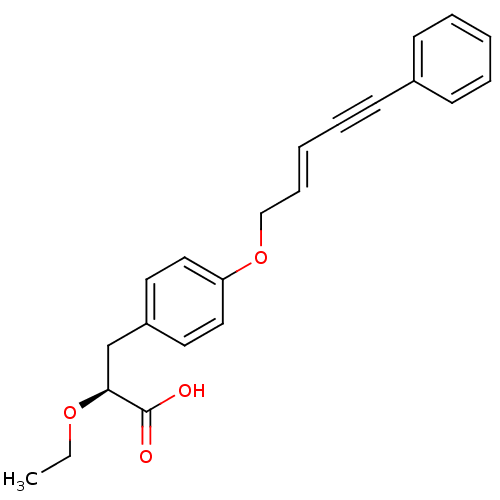
((S)-2-Ethoxy-3-[4-((E)-5-phenyl-pent-2-en-4-ynylox...)Show SMILES CCO[C@@H](Cc1ccc(OC\C=C\C#Cc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C22H22O4/c1-2-25-21(22(23)24)17-19-12-14-20(15-13-19)26-16-8-4-7-11-18-9-5-3-6-10-18/h3-6,8-10,12-15,21H,2,16-17H2,1H3,(H,23,24)/b8-4+/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation of rat Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 4883-94 (2003)
Article DOI: 10.1021/jm0309046
BindingDB Entry DOI: 10.7270/Q2222T5V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227689
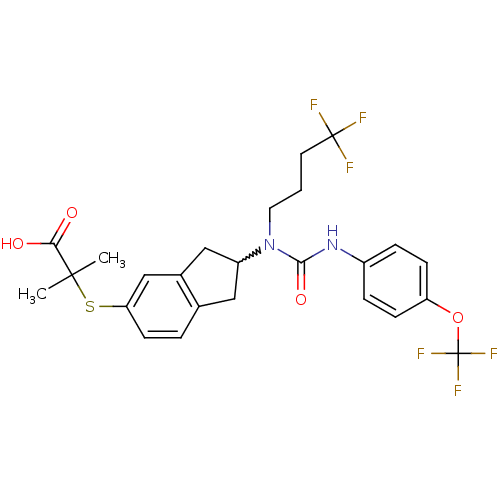
(2-methyl-2-(2-(1-(4,4,4-trifluorobutyl)-3-(4-(trif...)Show SMILES CC(C)(Sc1ccc2CC(Cc2c1)N(CCCC(F)(F)F)C(=O)Nc1ccc(OC(F)(F)F)cc1)C(O)=O |w:9.14| Show InChI InChI=1S/C25H26F6N2O4S/c1-23(2,21(34)35)38-20-9-4-15-12-18(13-16(15)14-20)33(11-3-10-24(26,27)28)22(36)32-17-5-7-19(8-6-17)37-25(29,30)31/h4-9,14,18H,3,10-13H2,1-2H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 707 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227700
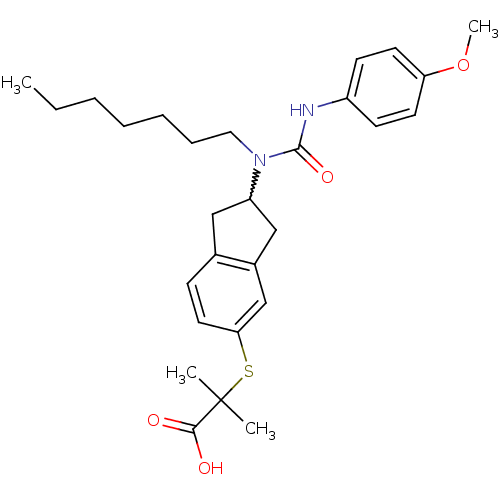
(2-(2-(1-heptyl-3-(4-methoxyphenyl)ureido)-2,3-dihy...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC)cc1 |w:8.7| Show InChI InChI=1S/C28H38N2O4S/c1-5-6-7-8-9-16-30(27(33)29-22-11-13-24(34-4)14-12-22)23-17-20-10-15-25(19-21(20)18-23)35-28(2,3)26(31)32/h10-15,19,23H,5-9,16-18H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 910 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227673
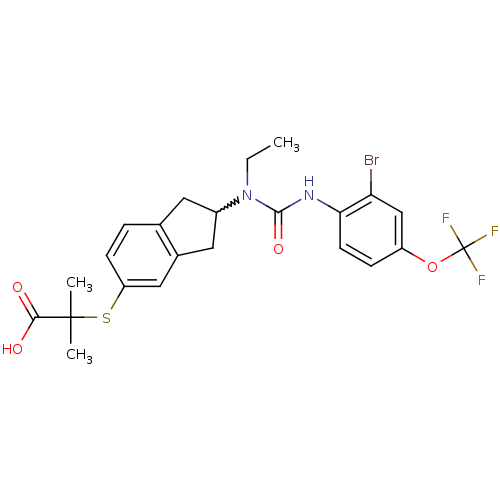
(2-(2-(3-(2-bromo-4-(trifluoromethoxy)phenyl)-1-eth...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1Br |w:3.2| Show InChI InChI=1S/C23H24BrF3N2O4S/c1-4-29(21(32)28-19-8-6-16(12-18(19)24)33-23(25,26)27)15-9-13-5-7-17(11-14(13)10-15)34-22(2,3)20(30)31/h5-8,11-12,15H,4,9-10H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227701
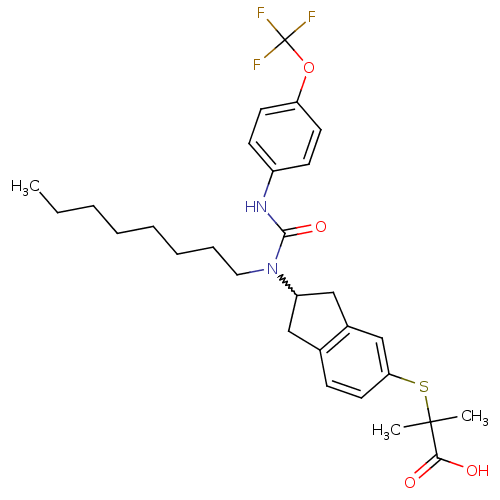
(2-methyl-2-(2-(1-octyl-3-(4-(trifluoromethoxy)phen...)Show SMILES CCCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:9.8| Show InChI InChI=1S/C29H37F3N2O4S/c1-4-5-6-7-8-9-16-34(27(37)33-22-11-13-24(14-12-22)38-29(30,31)32)23-17-20-10-15-25(19-21(20)18-23)39-28(2,3)26(35)36/h10-15,19,23H,4-9,16-18H2,1-3H3,(H,33,37)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227693
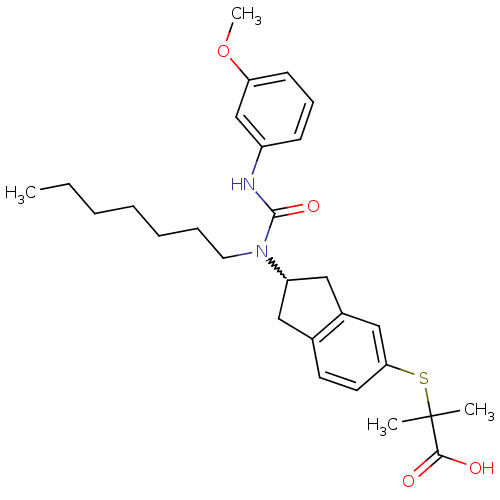
(2-(2-(1-heptyl-3-(3-methoxyphenyl)ureido)-2,3-dihy...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1cccc(OC)c1 |w:8.7| Show InChI InChI=1S/C28H38N2O4S/c1-5-6-7-8-9-15-30(27(33)29-22-11-10-12-24(19-22)34-4)23-16-20-13-14-25(18-21(20)17-23)35-28(2,3)26(31)32/h10-14,18-19,23H,5-9,15-17H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227670
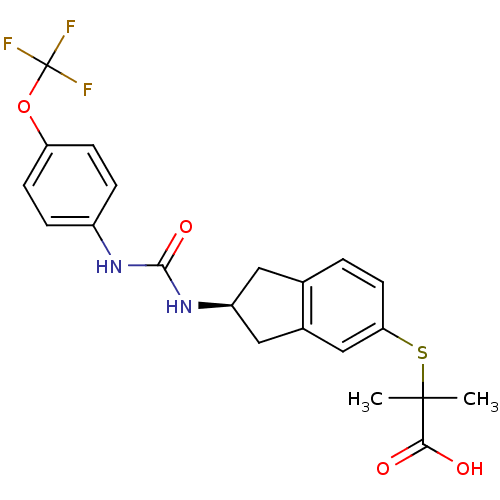
((S)-2-methyl-2-(2-(3-(4-(trifluoromethoxy)phenyl)u...)Show SMILES CC(C)(Sc1ccc2C[C@@H](Cc2c1)NC(=O)Nc1ccc(OC(F)(F)F)cc1)C(O)=O Show InChI InChI=1S/C21H21F3N2O4S/c1-20(2,18(27)28)31-17-8-3-12-9-15(10-13(12)11-17)26-19(29)25-14-4-6-16(7-5-14)30-21(22,23)24/h3-8,11,15H,9-10H2,1-2H3,(H,27,28)(H2,25,26,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227654
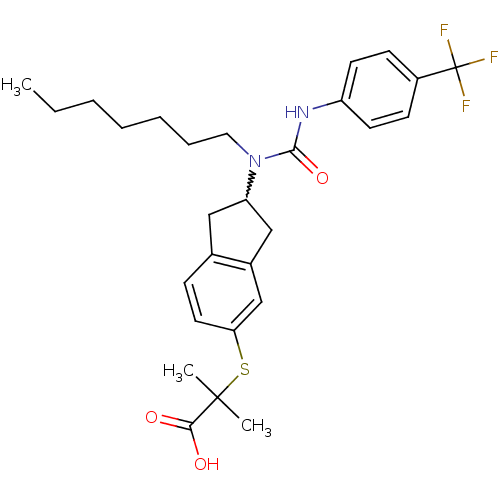
(2-(2-(1-heptyl-3-(4-(trifluoromethyl)phenyl)ureido...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(F)(F)F |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S/c1-4-5-6-7-8-15-33(26(36)32-22-12-10-21(11-13-22)28(29,30)31)23-16-19-9-14-24(18-20(19)17-23)37-27(2,3)25(34)35/h9-14,18,23H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227681
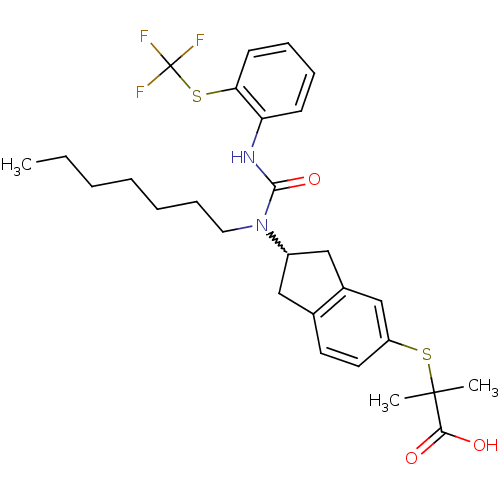
(2-(2-(1-heptyl-3-(2-(trifluoromethylthio)phenyl)ur...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccccc1SC(F)(F)F |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S2/c1-4-5-6-7-10-15-33(26(36)32-23-11-8-9-12-24(23)38-28(29,30)31)21-16-19-13-14-22(18-20(19)17-21)37-27(2,3)25(34)35/h8-9,11-14,18,21H,4-7,10,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227667
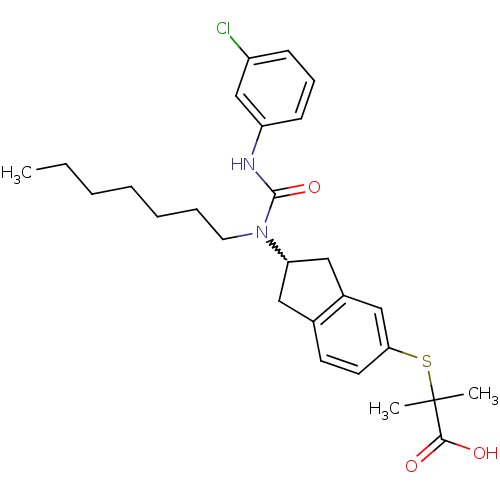
(2-(2-(3-(3-chlorophenyl)-1-heptylureido)-2,3-dihyd...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1cccc(Cl)c1 |w:8.7| Show InChI InChI=1S/C27H35ClN2O3S/c1-4-5-6-7-8-14-30(26(33)29-22-11-9-10-21(28)18-22)23-15-19-12-13-24(17-20(19)16-23)34-27(2,3)25(31)32/h9-13,17-18,23H,4-8,14-16H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM24566
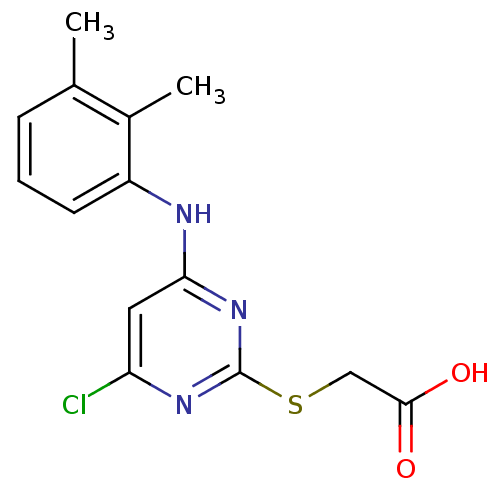
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro transactivation of rat Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 4883-94 (2003)
Article DOI: 10.1021/jm0309046
BindingDB Entry DOI: 10.7270/Q2222T5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50277897
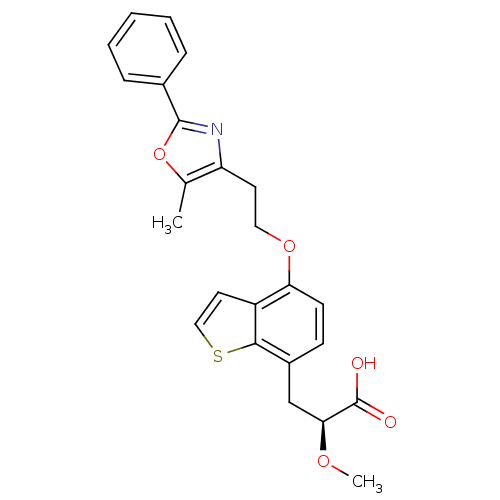
((2S)-2-methoxy-3-{4-[2-(5-methyl-2-phenyl-1,3-oxaz...)Show SMILES CO[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)c2ccsc12)C(O)=O |r| Show InChI InChI=1S/C24H23NO5S/c1-15-19(25-23(30-15)16-6-4-3-5-7-16)10-12-29-20-9-8-17(14-21(28-2)24(26)27)22-18(20)11-13-31-22/h3-9,11,13,21H,10,12,14H2,1-2H3,(H,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha |
Bioorg Med Chem Lett 19: 2468-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.036
BindingDB Entry DOI: 10.7270/Q2QC03C3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227698
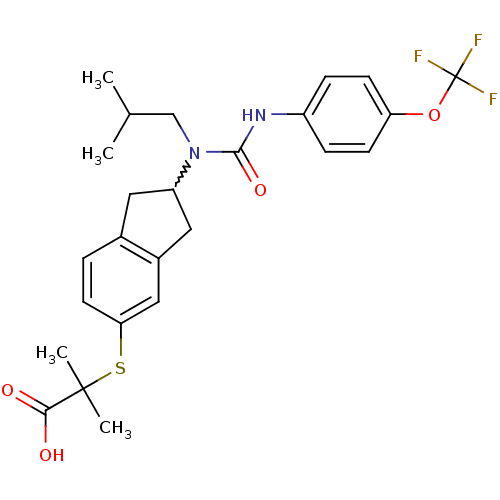
(2-(2-(1-isobutyl-3-(4-(trifluoromethoxy)phenyl)ure...)Show SMILES CC(C)CN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C25H29F3N2O4S/c1-15(2)14-30(23(33)29-18-6-8-20(9-7-18)34-25(26,27)28)19-11-16-5-10-21(13-17(16)12-19)35-24(3,4)22(31)32/h5-10,13,15,19H,11-12,14H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227665
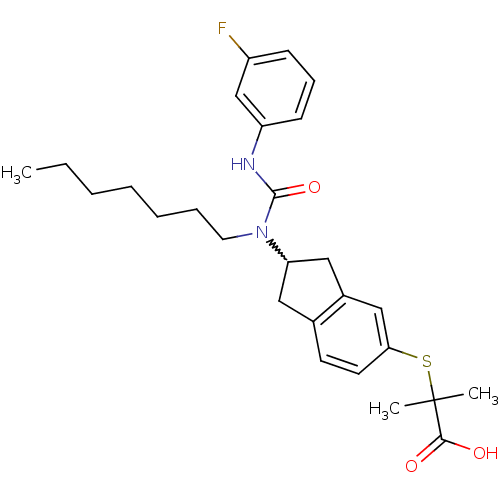
(2-(2-(3-(3-fluorophenyl)-1-heptylureido)-2,3-dihyd...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1cccc(F)c1 |w:8.7| Show InChI InChI=1S/C27H35FN2O3S/c1-4-5-6-7-8-14-30(26(33)29-22-11-9-10-21(28)18-22)23-15-19-12-13-24(17-20(19)16-23)34-27(2,3)25(31)32/h9-13,17-18,23H,4-8,14-16H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227706
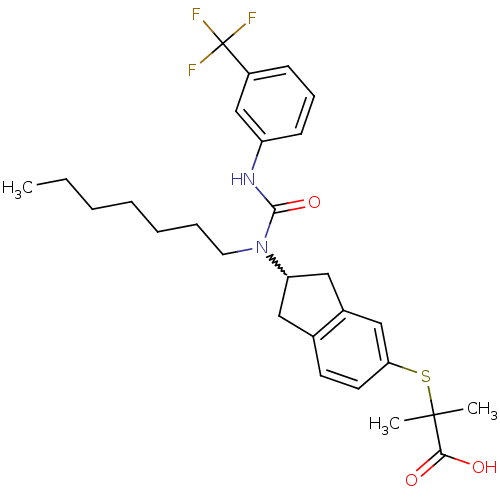
(2-(2-(1-heptyl-3-(3-(trifluoromethyl)phenyl)ureido...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1cccc(c1)C(F)(F)F |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S/c1-4-5-6-7-8-14-33(26(36)32-22-11-9-10-21(18-22)28(29,30)31)23-15-19-12-13-24(17-20(19)16-23)37-27(2,3)25(34)35/h9-13,17-18,23H,4-8,14-16H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data