 Found 5186 hits of ic50 for UniProtKB: P81908
Found 5186 hits of ic50 for UniProtKB: P81908 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Equus caballus (Horse)) | BDBM50599186
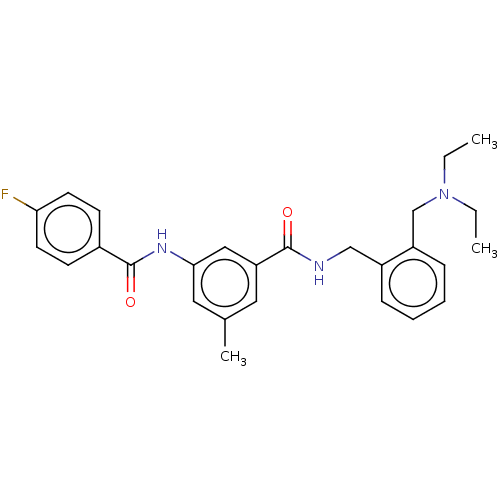
(CHEMBL5201089)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(C)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50393864
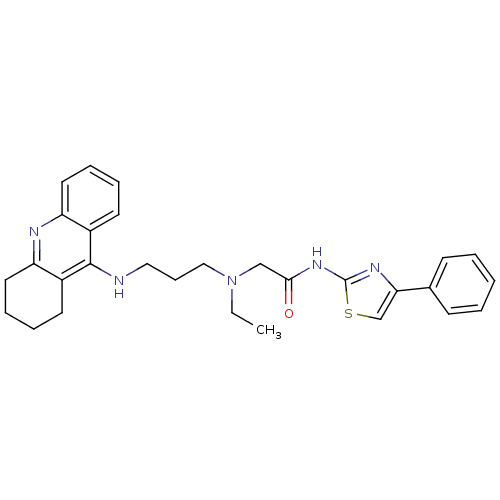
(CHEMBL2158116)Show SMILES CCN(CCCNc1c2CCCCc2nc2ccccc12)CC(=O)Nc1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C29H33N5OS/c1-2-34(19-27(35)33-29-32-26(20-36-29)21-11-4-3-5-12-21)18-10-17-30-28-22-13-6-8-15-24(22)31-25-16-9-7-14-23(25)28/h3-6,8,11-13,15,20H,2,7,9-10,14,16-19H2,1H3,(H,30,31)(H,32,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0447 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... |
Bioorg Med Chem 20: 6513-22 (2012)
Article DOI: 10.1016/j.bmc.2012.08.040
BindingDB Entry DOI: 10.7270/Q20Z74DZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sfax
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BuChE using butyrylthiocholine as substrate pretreated for 10 mins followed by substrate addition measured after 15 mins by... |
Eur J Med Chem 126: 576-589 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.050
BindingDB Entry DOI: 10.7270/Q21R6SR5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599172

(CHEMBL5181960)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(Br)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599154
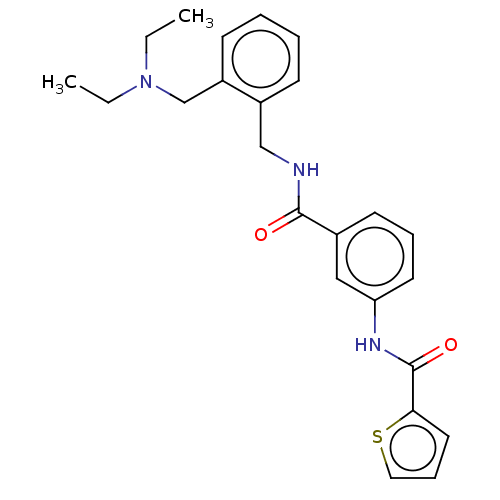
(CHEMBL5185039)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2cccs2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599188

(CHEMBL5184944)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599167
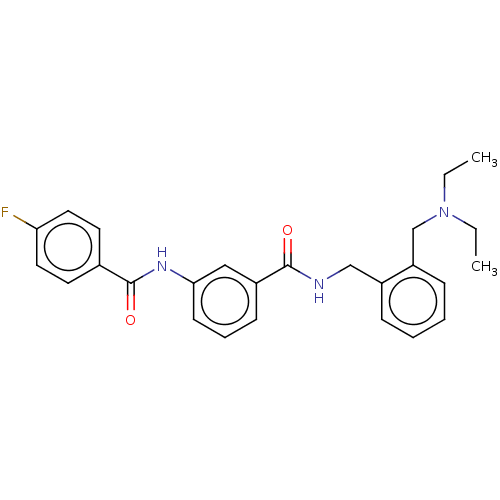
(CHEMBL5195228)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599191
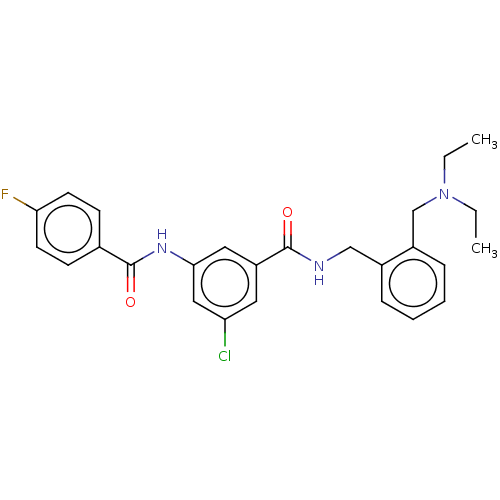
(CHEMBL5184728)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(Cl)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380559

(CHEMBL2019048)Show SMILES COc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O5/c1-42-23-19-28(39)32-29(40)21-31(43-30(32)20-23)34(41)37-17-11-7-5-3-2-4-6-10-16-36-33-24-12-8-9-13-26(24)38-27-18-22(35)14-15-25(27)33/h14-15,18-21,39H,2-13,16-17H2,1H3,(H,36,38)(H,37,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380537
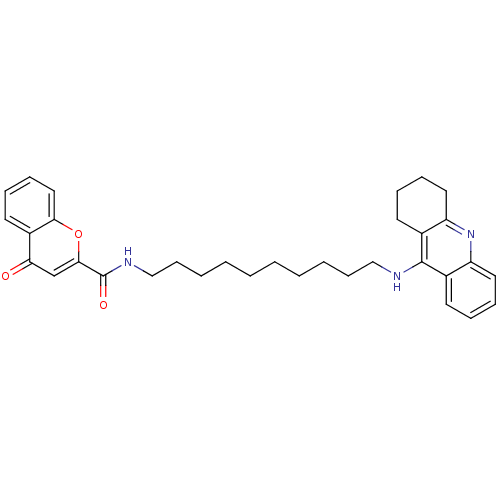
(CHEMBL2019030)Show SMILES O=C(NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C33H39N3O3/c37-29-23-31(39-30-20-12-9-17-26(29)30)33(38)35-22-14-6-4-2-1-3-5-13-21-34-32-24-15-7-10-18-27(24)36-28-19-11-8-16-25(28)32/h7,9-10,12,15,17-18,20,23H,1-6,8,11,13-14,16,19,21-22H2,(H,34,36)(H,35,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380541
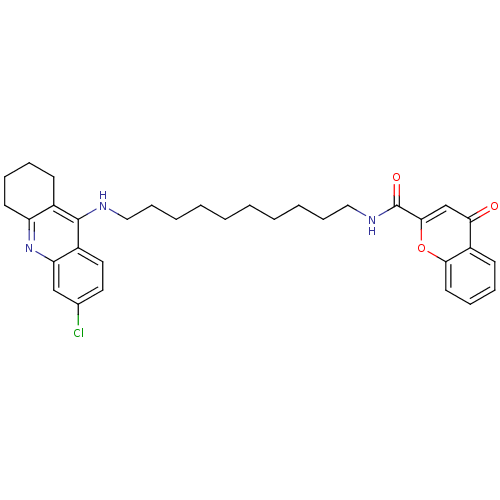
(CHEMBL2019032)Show SMILES Clc1ccc2c(NCCCCCCCCCCNC(=O)c3cc(=O)c4ccccc4o3)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H38ClN3O3/c34-23-17-18-25-28(21-23)37-27-15-9-7-13-24(27)32(25)35-19-11-5-3-1-2-4-6-12-20-36-33(39)31-22-29(38)26-14-8-10-16-30(26)40-31/h8,10,14,16-18,21-22H,1-7,9,11-13,15,19-20H2,(H,35,37)(H,36,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380536
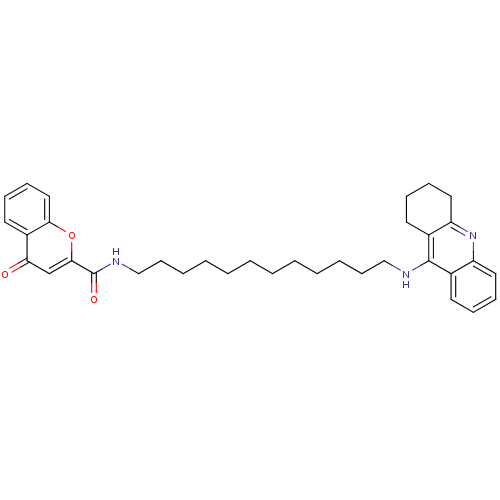
(CHEMBL2019031)Show SMILES O=C(NCCCCCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C35H43N3O3/c39-31-25-33(41-32-22-14-11-19-28(31)32)35(40)37-24-16-8-6-4-2-1-3-5-7-15-23-36-34-26-17-9-12-20-29(26)38-30-21-13-10-18-27(30)34/h9,11-12,14,17,19-20,22,25H,1-8,10,13,15-16,18,21,23-24H2,(H,36,38)(H,37,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599160

(CHEMBL5173939)Show SMILES CN(C)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2cccs2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380553
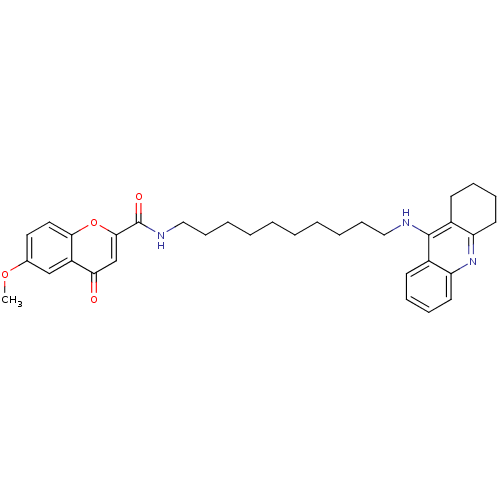
(CHEMBL2019034)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O4/c1-40-24-18-19-31-27(22-24)30(38)23-32(41-31)34(39)36-21-13-7-5-3-2-4-6-12-20-35-33-25-14-8-10-16-28(25)37-29-17-11-9-15-26(29)33/h8,10,14,16,18-19,22-23H,2-7,9,11-13,15,17,20-21H2,1H3,(H,35,37)(H,36,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380542
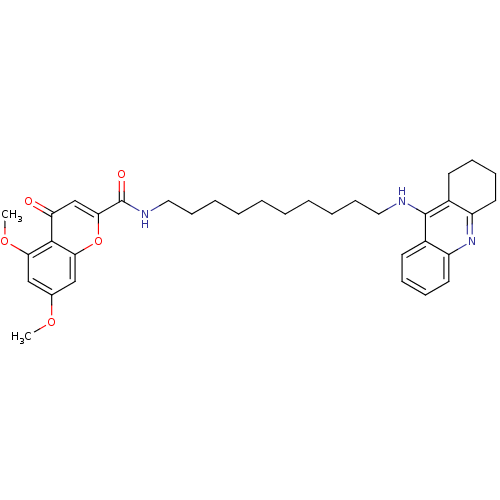
(CHEMBL2019037)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-24-21-30(42-2)33-29(39)23-32(43-31(33)22-24)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-25-15-9-11-17-27(25)38-28-18-12-10-16-26(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599173

(CHEMBL5200785)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(I)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380546
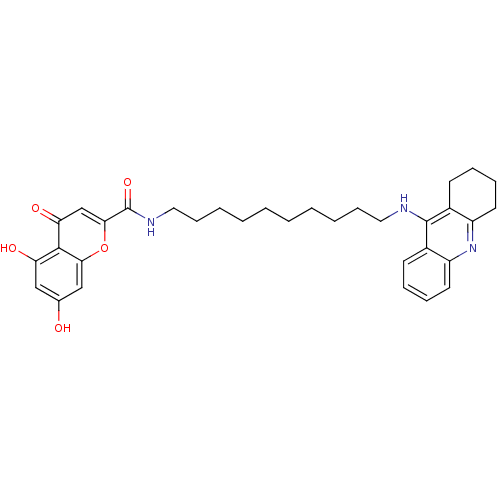
(CHEMBL2019047)Show SMILES Oc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H39N3O5/c37-22-19-27(38)31-28(39)21-30(41-29(31)20-22)33(40)35-18-12-6-4-2-1-3-5-11-17-34-32-23-13-7-9-15-25(23)36-26-16-10-8-14-24(26)32/h7,9,13,15,19-21,37-38H,1-6,8,10-12,14,16-18H2,(H,34,36)(H,35,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50393868
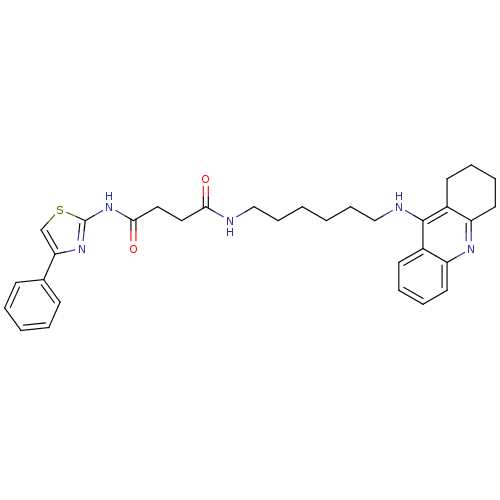
(CHEMBL2158112)Show SMILES O=C(CCC(=O)Nc1nc(cs1)-c1ccccc1)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C32H37N5O2S/c38-29(18-19-30(39)37-32-36-28(22-40-32)23-12-4-3-5-13-23)33-20-10-1-2-11-21-34-31-24-14-6-8-16-26(24)35-27-17-9-7-15-25(27)31/h3-6,8,12-14,16,22H,1-2,7,9-11,15,17-21H2,(H,33,38)(H,34,35)(H,36,37,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... |
Bioorg Med Chem 20: 6513-22 (2012)
Article DOI: 10.1016/j.bmc.2012.08.040
BindingDB Entry DOI: 10.7270/Q20Z74DZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599185
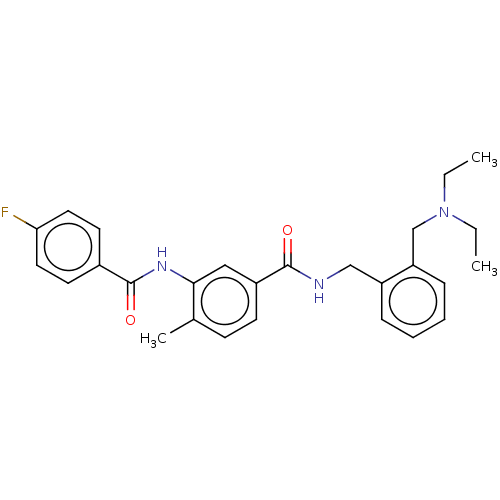
(CHEMBL5199323)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1ccc(C)c(NC(=O)c2ccc(F)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599182
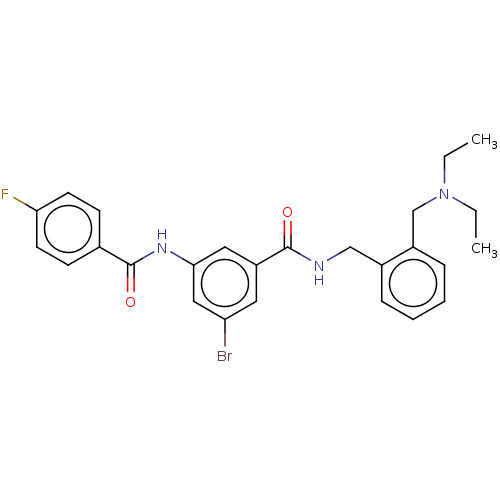
(CHEMBL5197490)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(Br)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380552
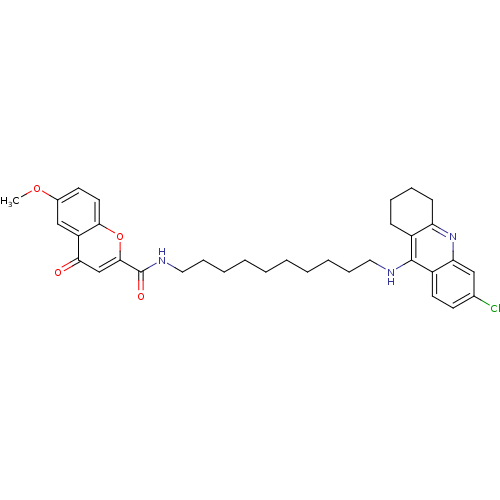
(CHEMBL2019035)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O4/c1-41-24-15-17-31-27(21-24)30(39)22-32(42-31)34(40)37-19-11-7-5-3-2-4-6-10-18-36-33-25-12-8-9-13-28(25)38-29-20-23(35)14-16-26(29)33/h14-17,20-22H,2-13,18-19H2,1H3,(H,36,38)(H,37,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599161
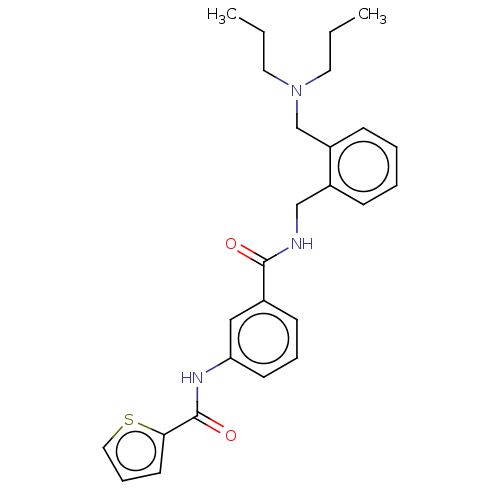
(CHEMBL5208367)Show SMILES CCCN(CCC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2cccs2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50393858

(CHEMBL2158122)Show SMILES CCN(CCCNc1c2CCCCc2nc2ccccc12)CCC(=O)Nc1nc(cs1)-c1ccc(OC)cc1 Show InChI InChI=1S/C31H37N5O2S/c1-3-36(20-17-29(37)35-31-34-28(21-39-31)22-13-15-23(38-2)16-14-22)19-8-18-32-30-24-9-4-6-11-26(24)33-27-12-7-5-10-25(27)30/h4,6,9,11,13-16,21H,3,5,7-8,10,12,17-20H2,1-2H3,(H,32,33)(H,34,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... |
Bioorg Med Chem 20: 6513-22 (2012)
Article DOI: 10.1016/j.bmc.2012.08.040
BindingDB Entry DOI: 10.7270/Q20Z74DZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599170
(CHEMBL5201163)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2c(F)c(F)c(F)c(F)c2F)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50456821

(CHEMBL4208961)Show SMILES [Br-].Cc1cc(=O)oc2cc(OCc3cc[n+](Cc4ccccc4Br)cc3)ccc12 Show InChI InChI=1S/C23H19BrNO3/c1-16-12-23(26)28-22-13-19(6-7-20(16)22)27-15-17-8-10-25(11-9-17)14-18-4-2-3-5-21(18)24/h2-13H,14-15H2,1H3/q+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... |
Eur J Med Chem 139: 48-59 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.055
BindingDB Entry DOI: 10.7270/Q2GM89X1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380539
(CHEMBL2019028)Show SMILES O=C(NCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C31H35N3O3/c35-27-21-29(37-28-18-10-7-15-24(27)28)31(36)33-20-12-4-2-1-3-11-19-32-30-22-13-5-8-16-25(22)34-26-17-9-6-14-23(26)30/h5,7-8,10,13,15-16,18,21H,1-4,6,9,11-12,14,17,19-20H2,(H,32,34)(H,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50114361
(CHEMBL3604192)Show SMILES Cl.CN(C)c1ccc(cc1)C(=O)NCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C24H28N4O.ClH/c1-28(2)18-13-11-17(12-14-18)24(29)26-16-15-25-23-19-7-3-5-9-21(19)27-22-10-6-4-8-20(22)23;/h3,5,7,9,11-14H,4,6,8,10,15-16H2,1-2H3,(H,25,27)(H,26,29);1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay |
Bioorg Med Chem 23: 5610-8 (2015)
Article DOI: 10.1016/j.bmc.2015.07.029
BindingDB Entry DOI: 10.7270/Q25T3N9G |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50317179
(2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...)Show SMILES C[n+]1ccc2c(c1)n(CCCCCCCCCn1c3ccccc3c3cc[n+](C)cc13)c1ccccc21 Show InChI InChI=1S/C33H38N4/c1-34-22-18-28-26-14-8-10-16-30(26)36(32(28)24-34)20-12-6-4-3-5-7-13-21-37-31-17-11-9-15-27(31)29-19-23-35(2)25-33(29)37/h8-11,14-19,22-25H,3-7,12-13,20-21H2,1-2H3/q+2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.507 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE by modified Ellman's method |
J Med Chem 53: 3611-7 (2010)
Article DOI: 10.1021/jm1000024
BindingDB Entry DOI: 10.7270/Q2SJ1KS0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599175
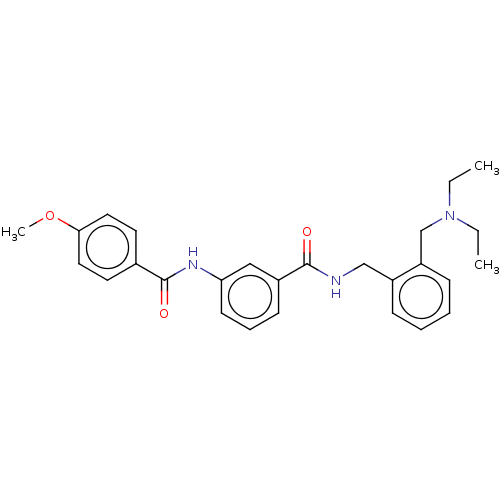
(CHEMBL5188290)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(OC)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599190
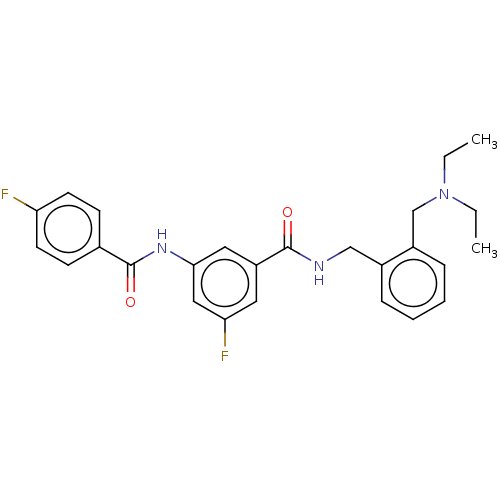
(CHEMBL5191236)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(F)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380543
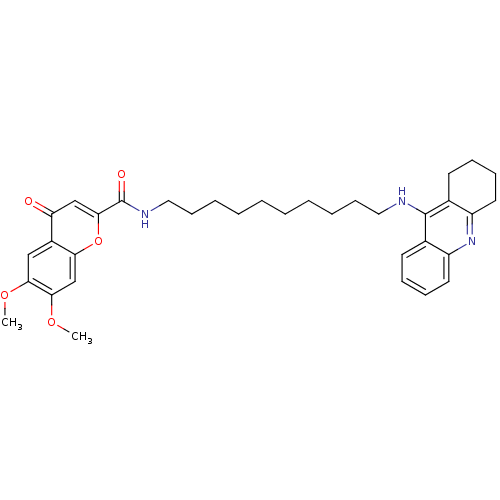
(CHEMBL2019040)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-31-21-26-29(39)22-33(43-30(26)23-32(31)42-2)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-24-15-9-11-17-27(24)38-28-18-12-10-16-25(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50393867

(CHEMBL2158113)Show SMILES O=C(CCCC(=O)Nc1nc(cs1)-c1ccccc1)NCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H35N5O2S/c37-28(17-10-18-29(38)36-31-35-27(21-39-31)22-11-2-1-3-12-22)32-19-8-9-20-33-30-23-13-4-6-15-25(23)34-26-16-7-5-14-24(26)30/h1-4,6,11-13,15,21H,5,7-10,14,16-20H2,(H,32,37)(H,33,34)(H,35,36,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... |
Bioorg Med Chem 20: 6513-22 (2012)
Article DOI: 10.1016/j.bmc.2012.08.040
BindingDB Entry DOI: 10.7270/Q20Z74DZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380538
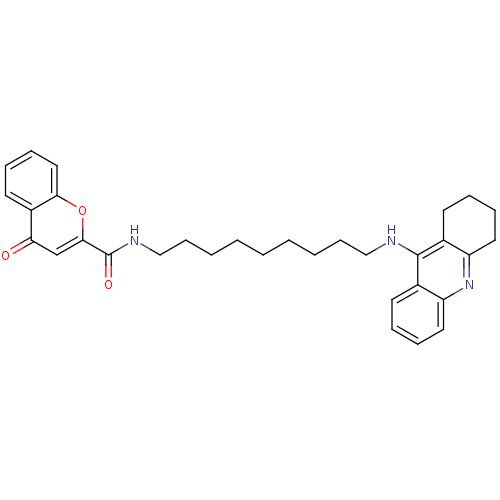
(CHEMBL2019029)Show SMILES O=C(NCCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C32H37N3O3/c36-28-22-30(38-29-19-11-8-16-25(28)29)32(37)34-21-13-5-3-1-2-4-12-20-33-31-23-14-6-9-17-26(23)35-27-18-10-7-15-24(27)31/h6,8-9,11,14,16-17,19,22H,1-5,7,10,12-13,15,18,20-21H2,(H,33,35)(H,34,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50433319
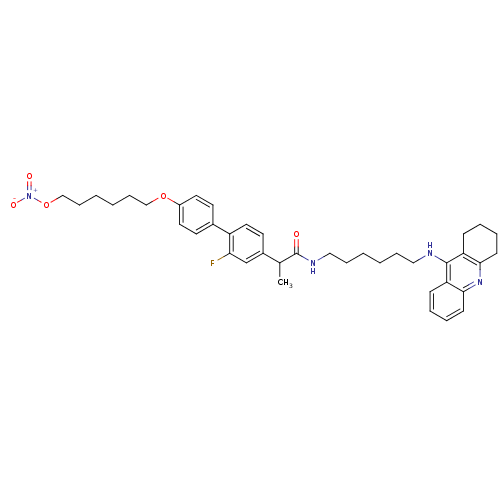
(CHEMBL2376477)Show SMILES CC(C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12)c1ccc(c(F)c1)-c1ccc(OCCCCCCO[N+]([O-])=O)cc1 Show InChI InChI=1S/C40H49FN4O5/c1-29(31-20-23-33(36(41)28-31)30-18-21-32(22-19-30)49-26-12-4-5-13-27-50-45(47)48)40(46)43-25-11-3-2-10-24-42-39-34-14-6-8-16-37(34)44-38-17-9-7-15-35(38)39/h6,8,14,16,18-23,28-29H,2-5,7,9-13,15,17,24-27H2,1H3,(H,42,44)(H,43,46) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using acetylcholine as substrate preincubated for 5 mins prior to substrate addition measured after 2 mins by Ellman'... |
Bioorg Med Chem 21: 2462-70 (2013)
Article DOI: 10.1016/j.bmc.2013.03.005
BindingDB Entry DOI: 10.7270/Q2319X82 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50393870

(CHEMBL2158110)Show SMILES O=C(CCC(=O)Nc1nc(cs1)-c1ccccc1)NCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N5O2S/c36-27(16-17-28(37)35-30-34-26(20-38-30)21-10-2-1-3-11-21)31-18-8-9-19-32-29-22-12-4-6-14-24(22)33-25-15-7-5-13-23(25)29/h1-4,6,10-12,14,20H,5,7-9,13,15-19H2,(H,31,36)(H,32,33)(H,34,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... |
Bioorg Med Chem 20: 6513-22 (2012)
Article DOI: 10.1016/j.bmc.2012.08.040
BindingDB Entry DOI: 10.7270/Q20Z74DZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50114368
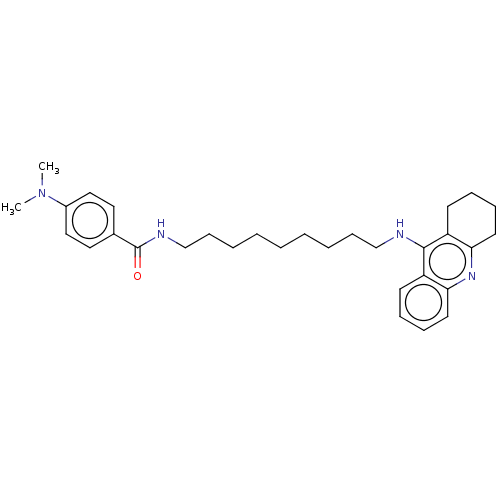
(CHEMBL3604199)Show SMILES Cl.CN(C)c1ccc(cc1)C(=O)NCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H42N4O.ClH/c1-35(2)25-20-18-24(19-21-25)31(36)33-23-13-7-5-3-4-6-12-22-32-30-26-14-8-10-16-28(26)34-29-17-11-9-15-27(29)30;/h8,10,14,16,18-21H,3-7,9,11-13,15,17,22-23H2,1-2H3,(H,32,34)(H,33,36);1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay |
Bioorg Med Chem 23: 5610-8 (2015)
Article DOI: 10.1016/j.bmc.2015.07.029
BindingDB Entry DOI: 10.7270/Q25T3N9G |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50114367
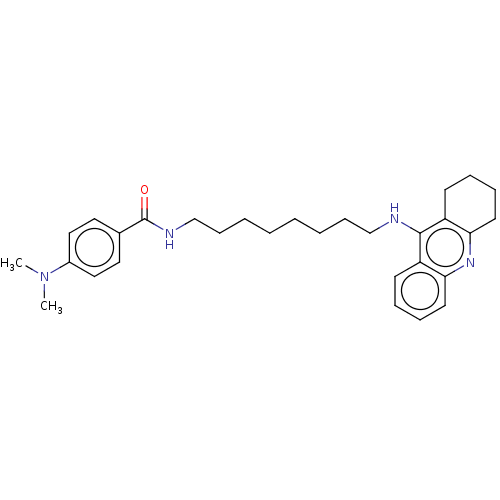
(CHEMBL3604198)Show SMILES Cl.CN(C)c1ccc(cc1)C(=O)NCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H40N4O.ClH/c1-34(2)24-19-17-23(18-20-24)30(35)32-22-12-6-4-3-5-11-21-31-29-25-13-7-9-15-27(25)33-28-16-10-8-14-26(28)29;/h7,9,13,15,17-20H,3-6,8,10-12,14,16,21-22H2,1-2H3,(H,31,33)(H,32,35);1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay |
Bioorg Med Chem 23: 5610-8 (2015)
Article DOI: 10.1016/j.bmc.2015.07.029
BindingDB Entry DOI: 10.7270/Q25T3N9G |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380562
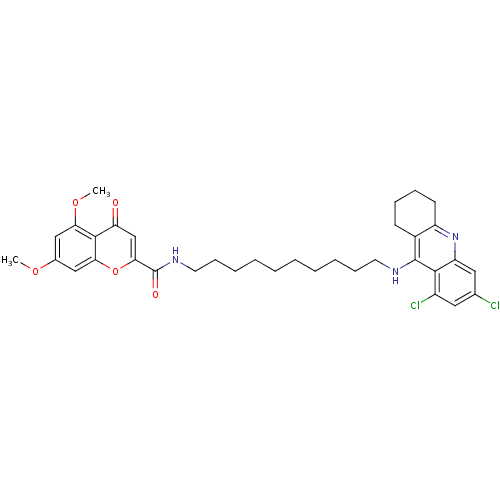
(CHEMBL2019039)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)cc(Cl)c12 Show InChI InChI=1S/C35H41Cl2N3O5/c1-43-23-19-29(44-2)33-28(41)21-31(45-30(33)20-23)35(42)39-16-12-8-6-4-3-5-7-11-15-38-34-24-13-9-10-14-26(24)40-27-18-22(36)17-25(37)32(27)34/h17-21H,3-16H2,1-2H3,(H,38,40)(H,39,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599164
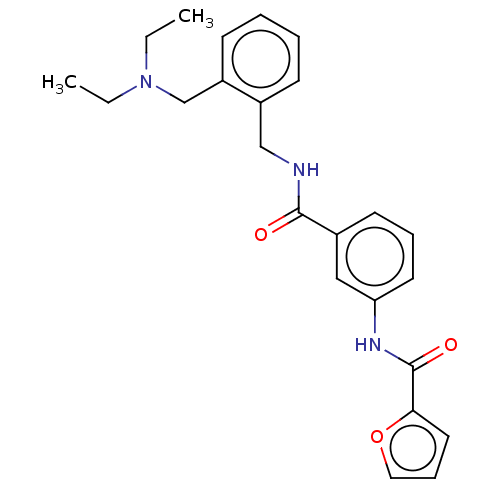
(CHEMBL5201951)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccco2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599189
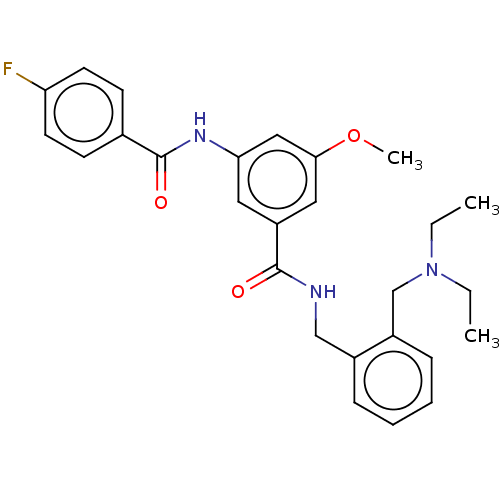
(CHEMBL5183552)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(NC(=O)c2ccc(F)cc2)cc(OC)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380550
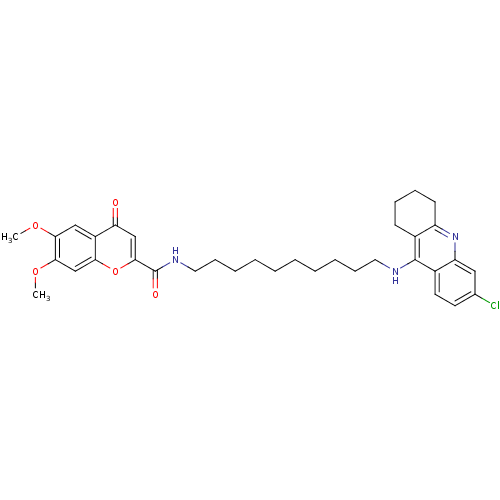
(CHEMBL2019041)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H42ClN3O5/c1-42-31-20-26-29(40)21-33(44-30(26)22-32(31)43-2)35(41)38-18-12-8-6-4-3-5-7-11-17-37-34-24-13-9-10-14-27(24)39-28-19-23(36)15-16-25(28)34/h15-16,19-22H,3-14,17-18H2,1-2H3,(H,37,39)(H,38,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
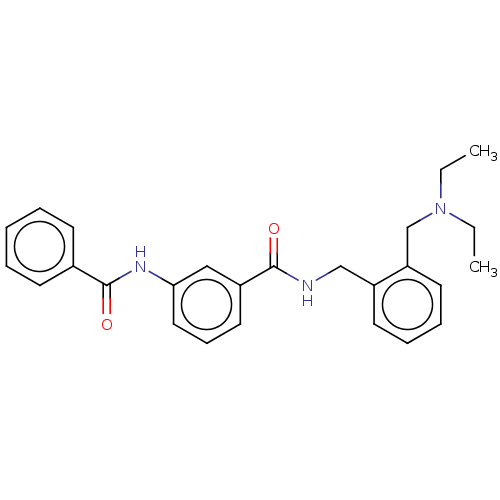
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599166
(CHEMBL5195383)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccccc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396122

(CHEMBL2171326)Show SMILES COc1cc(\C=C\C(=O)NCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc1OCCCO[N+]([O-])=O Show InChI InChI=1S/C34H44N4O6/c1-42-32-25-26(17-19-31(32)43-23-12-24-44-38(40)41)18-20-33(39)35-21-10-4-2-3-5-11-22-36-34-27-13-6-8-15-29(27)37-30-16-9-7-14-28(30)34/h6,8,13,15,17-20,25H,2-5,7,9-12,14,16,21-24H2,1H3,(H,35,39)(H,36,37)/b20-18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method |
J Med Chem 55: 4309-21 (2012)
Article DOI: 10.1021/jm300106z
BindingDB Entry DOI: 10.7270/Q21837N7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380544
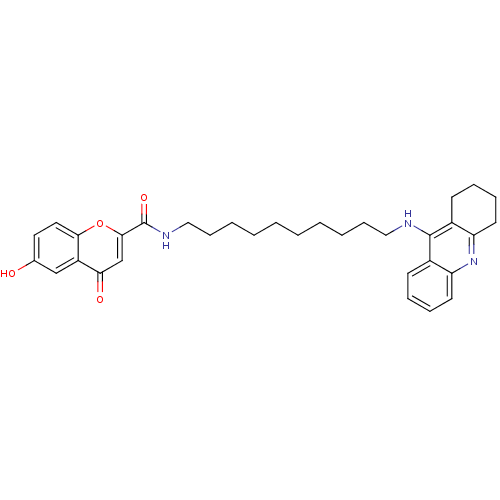
(CHEMBL2019043)Show SMILES Oc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H39N3O4/c37-23-17-18-30-26(21-23)29(38)22-31(40-30)33(39)35-20-12-6-4-2-1-3-5-11-19-34-32-24-13-7-9-15-27(24)36-28-16-10-8-14-25(28)32/h7,9,13,15,17-18,21-22,37H,1-6,8,10-12,14,16,19-20H2,(H,34,36)(H,35,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50433318
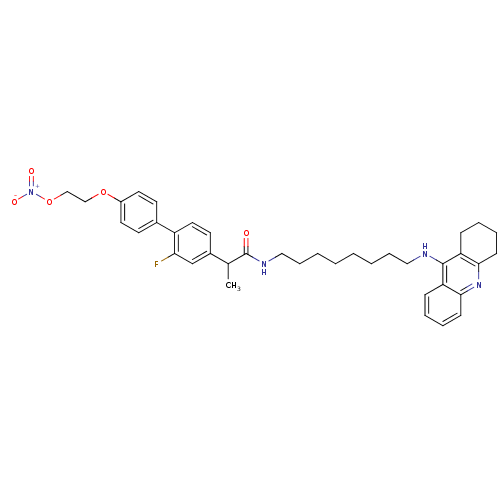
(CHEMBL2376478)Show SMILES CC(C(=O)NCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1ccc(c(F)c1)-c1ccc(OCCO[N+]([O-])=O)cc1 Show InChI InChI=1S/C38H45FN4O5/c1-27(29-18-21-31(34(39)26-29)28-16-19-30(20-17-28)47-24-25-48-43(45)46)38(44)41-23-11-5-3-2-4-10-22-40-37-32-12-6-8-14-35(32)42-36-15-9-7-13-33(36)37/h6,8,12,14,16-21,26-27H,2-5,7,9-11,13,15,22-25H2,1H3,(H,40,42)(H,41,44) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using acetylcholine as substrate preincubated for 5 mins prior to substrate addition measured after 2 mins by Ellman'... |
Bioorg Med Chem 21: 2462-70 (2013)
Article DOI: 10.1016/j.bmc.2013.03.005
BindingDB Entry DOI: 10.7270/Q2319X82 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380551
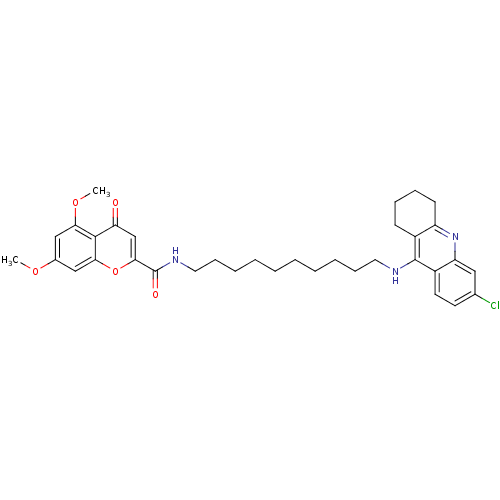
(CHEMBL2019038)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H42ClN3O5/c1-42-24-20-30(43-2)33-29(40)22-32(44-31(33)21-24)35(41)38-18-12-8-6-4-3-5-7-11-17-37-34-25-13-9-10-14-27(25)39-28-19-23(36)15-16-26(28)34/h15-16,19-22H,3-14,17-18H2,1-2H3,(H,37,39)(H,38,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599171
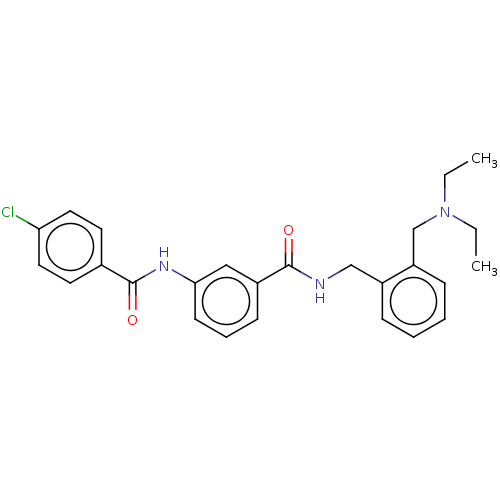
(CHEMBL5198895)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(Cl)cc2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50114365
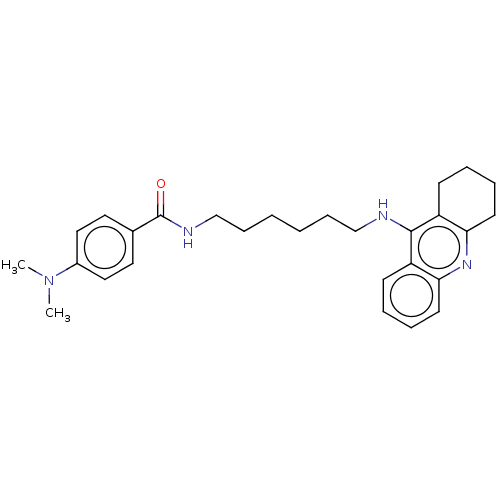
(CHEMBL3604196)Show SMILES Cl.CN(C)c1ccc(cc1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C28H36N4O.ClH/c1-32(2)22-17-15-21(16-18-22)28(33)30-20-10-4-3-9-19-29-27-23-11-5-7-13-25(23)31-26-14-8-6-12-24(26)27;/h5,7,11,13,15-18H,3-4,6,8-10,12,14,19-20H2,1-2H3,(H,29,31)(H,30,33);1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay |
Bioorg Med Chem 23: 5610-8 (2015)
Article DOI: 10.1016/j.bmc.2015.07.029
BindingDB Entry DOI: 10.7270/Q25T3N9G |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50599178
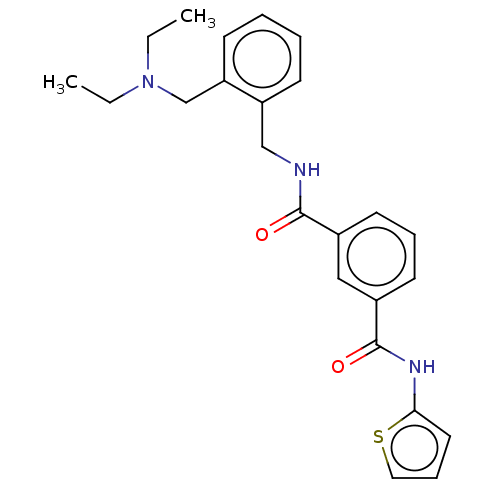
(CHEMBL5190491)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(c1)C(=O)Nc1cccs1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate incubated for 15 mins followed by substrate addition by Ellman's method |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data