 Found 195 hits of ic50 for UniProtKB: P41240
Found 195 hits of ic50 for UniProtKB: P41240 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Silesia in Katowice
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay |
Eur J Med Chem 163: 610-625 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.012
BindingDB Entry DOI: 10.7270/Q29P353V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Silesia in Katowice
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay |
Eur J Med Chem 163: 610-625 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.012
BindingDB Entry DOI: 10.7270/Q29P353V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50305126
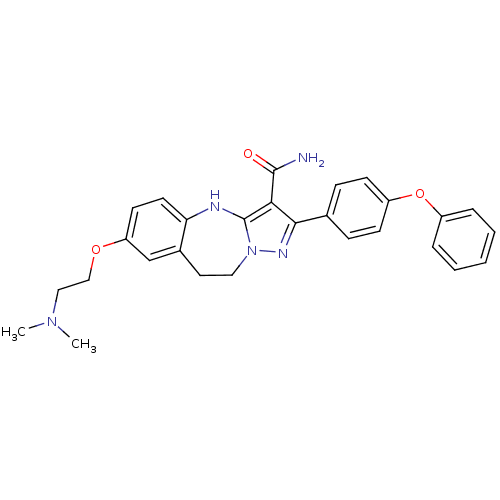
(7-(2-(dimethylamino)ethoxy)-2-(4-phenoxyphenyl)-9,...)Show SMILES CN(C)CCOc1ccc2Nc3c(C(N)=O)c(nn3CCc2c1)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C28H29N5O3/c1-32(2)16-17-35-23-12-13-24-20(18-23)14-15-33-28(30-24)25(27(29)34)26(31-33)19-8-10-22(11-9-19)36-21-6-4-3-5-7-21/h3-13,18,30H,14-17H2,1-2H3,(H2,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Csk |
Bioorg Med Chem Lett 20: 112-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.013
BindingDB Entry DOI: 10.7270/Q22Z15NV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503098
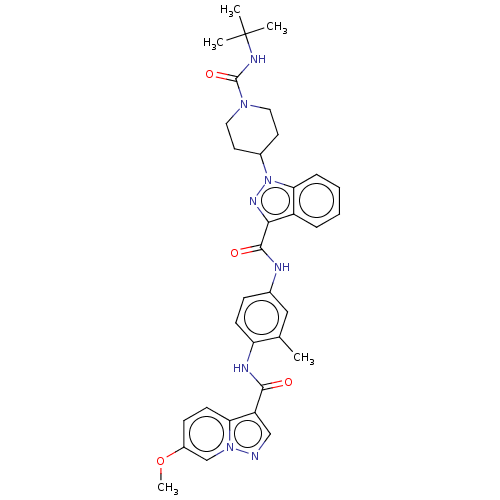
(CHEMBL4582324)Show SMILES COc1ccc2c(cnn2c1)C(=O)Nc1ccc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccccc23)cc1C Show InChI InChI=1S/C34H38N8O4/c1-21-18-22(10-12-27(21)37-31(43)26-19-35-41-20-24(46-5)11-13-28(26)41)36-32(44)30-25-8-6-7-9-29(25)42(39-30)23-14-16-40(17-15-23)33(45)38-34(2,3)4/h6-13,18-20,23H,14-17H2,1-5H3,(H,36,44)(H,37,43)(H,38,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) using fluorescent labeled peptide as substrate in presence of ATP at Km concentration by caliper method |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... |
US Patent US9181263 (2015)
BindingDB Entry DOI: 10.7270/Q2765D5Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... |
US Patent US9278100 (2016)
BindingDB Entry DOI: 10.7270/Q20C4TMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CSK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.12.079
BindingDB Entry DOI: 10.7270/Q2P272TR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM97672

(US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NC(=O)C=C |r,wU:23.26,26.33,(-3.76,2.88,;-3.76,1.34,;-5.09,.57,;-5.09,-.97,;-3.76,-1.74,;-2.43,-.97,;-.96,-1.45,;-.06,-.2,;-.96,1.05,;-.56,2.53,;.92,2.93,;1.32,4.42,;.23,5.51,;.63,7,;2.12,7.4,;3.21,6.31,;4.7,6.7,;5.09,8.19,;4.01,9.28,;2.52,8.88,;-1.25,5.11,;-1.65,3.62,;-2.43,.57,;-.56,-2.93,;-1.65,-4.02,;-1.25,-5.51,;.23,-5.91,;1.32,-4.82,;.92,-3.33,;.63,-7.4,;2.12,-7.79,;2.52,-9.28,;3.21,-6.7,;4.7,-7.1,)| Show InChI InChI=1S/C26H26N6O2/c1-2-22(33)30-18-10-12-19(13-11-18)32-26-23(25(27)28-16-29-26)24(31-32)17-8-14-21(15-9-17)34-20-6-4-3-5-7-20/h2-9,14-16,18-19H,1,10-13H2,(H,30,33)(H2,27,28,29)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... |
US Patent US9181263 (2015)
BindingDB Entry DOI: 10.7270/Q2765D5Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM97672

(US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CC[C@@H](CC1)NC(=O)C=C |r,wU:23.26,26.33,(-3.76,2.88,;-3.76,1.34,;-5.09,.57,;-5.09,-.97,;-3.76,-1.74,;-2.43,-.97,;-.96,-1.45,;-.06,-.2,;-.96,1.05,;-.56,2.53,;.92,2.93,;1.32,4.42,;.23,5.51,;.63,7,;2.12,7.4,;3.21,6.31,;4.7,6.7,;5.09,8.19,;4.01,9.28,;2.52,8.88,;-1.25,5.11,;-1.65,3.62,;-2.43,.57,;-.56,-2.93,;-1.65,-4.02,;-1.25,-5.51,;.23,-5.91,;1.32,-4.82,;.92,-3.33,;.63,-7.4,;2.12,-7.79,;2.52,-9.28,;3.21,-6.7,;4.7,-7.1,)| Show InChI InChI=1S/C26H26N6O2/c1-2-22(33)30-18-10-12-19(13-11-18)32-26-23(25(27)28-16-29-26)24(31-32)17-8-14-21(15-9-17)34-20-6-4-3-5-7-20/h2-9,14-16,18-19H,1,10-13H2,(H,30,33)(H2,27,28,29)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC
US Patent
| Assay Description
IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... |
US Patent US9278100 (2016)
BindingDB Entry DOI: 10.7270/Q20C4TMX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503095
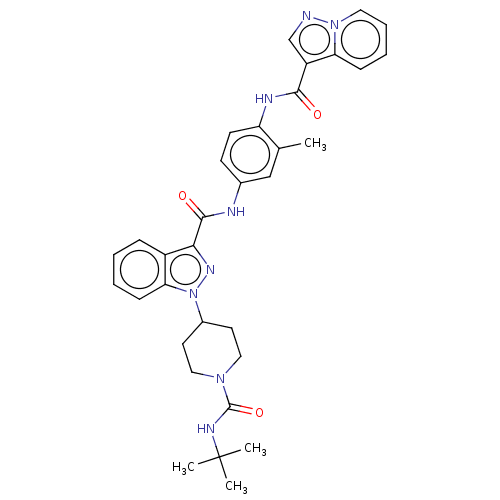
(CHEMBL4577049)Show SMILES Cc1cc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccccc23)ccc1NC(=O)c1cnn2ccccc12 Show InChI InChI=1S/C33H36N8O3/c1-21-19-22(12-13-26(21)36-30(42)25-20-34-40-16-8-7-10-27(25)40)35-31(43)29-24-9-5-6-11-28(24)41(38-29)23-14-17-39(18-15-23)32(44)37-33(2,3)4/h5-13,16,19-20,23H,14-15,17-18H2,1-4H3,(H,35,43)(H,36,42)(H,37,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503098

(CHEMBL4582324)Show SMILES COc1ccc2c(cnn2c1)C(=O)Nc1ccc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccccc23)cc1C Show InChI InChI=1S/C34H38N8O4/c1-21-18-22(10-12-27(21)37-31(43)26-19-35-41-20-24(46-5)11-13-28(26)41)36-32(44)30-25-8-6-7-9-29(25)42(39-30)23-14-16-40(17-15-23)33(45)38-34(2,3)4/h6-13,18-20,23H,14-17H2,1-5H3,(H,36,44)(H,37,43)(H,38,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503102
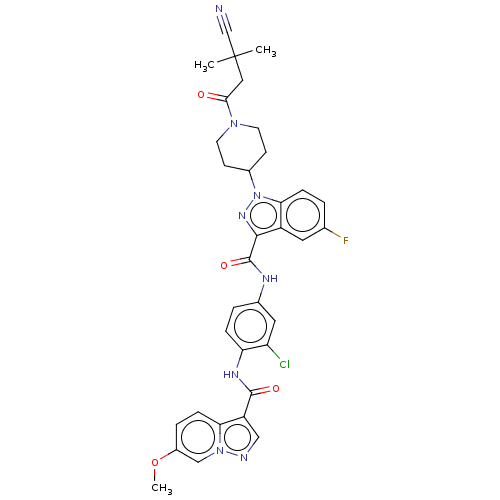
(CHEMBL4445994)Show SMILES COc1ccc2c(cnn2c1)C(=O)Nc1ccc(NC(=O)c2nn(C3CCN(CC3)C(=O)CC(C)(C)C#N)c3ccc(F)cc23)cc1Cl Show InChI InChI=1S/C34H32ClFN8O4/c1-34(2,19-37)16-30(45)42-12-10-22(11-13-42)44-29-8-4-20(36)14-24(29)31(41-44)33(47)39-21-5-7-27(26(35)15-21)40-32(46)25-17-38-43-18-23(48-3)6-9-28(25)43/h4-9,14-15,17-18,22H,10-13,16H2,1-3H3,(H,39,47)(H,40,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503099
(CHEMBL4545269)Show SMILES Cc1cc(NC(=O)c2nn(C3CCN(CC3)C(=O)CC(C)(C)C#N)c3ccccc23)ccc1NC(=O)c1cnn2ccccc12 Show InChI InChI=1S/C34H34N8O3/c1-22-18-23(11-12-27(22)38-32(44)26-20-36-41-15-7-6-9-28(26)41)37-33(45)31-25-8-4-5-10-29(25)42(39-31)24-13-16-40(17-14-24)30(43)19-34(2,3)21-35/h4-12,15,18,20,24H,13-14,16-17,19H2,1-3H3,(H,37,45)(H,38,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503099

(CHEMBL4545269)Show SMILES Cc1cc(NC(=O)c2nn(C3CCN(CC3)C(=O)CC(C)(C)C#N)c3ccccc23)ccc1NC(=O)c1cnn2ccccc12 Show InChI InChI=1S/C34H34N8O3/c1-22-18-23(11-12-27(22)38-32(44)26-20-36-41-15-7-6-9-28(26)41)37-33(45)31-25-8-4-5-10-29(25)42(39-31)24-13-16-40(17-14-24)30(43)19-34(2,3)21-35/h4-12,15,18,20,24H,13-14,16-17,19H2,1-3H3,(H,37,45)(H,38,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) using fluorescent labeled peptide as substrate in presence of ATP at Km concentration by caliper method |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503100

(CHEMBL4443654)Show SMILES CC(C)(C)NC(=O)N1CCC(CC1)n1nc(C(=O)Nc2ccc(NC(=O)c3cnn4ccccc34)c(Cl)c2)c2ccccc12 Show InChI InChI=1S/C32H33ClN8O3/c1-32(2,3)37-31(44)39-16-13-21(14-17-39)41-27-10-5-4-8-22(27)28(38-41)30(43)35-20-11-12-25(24(33)18-20)36-29(42)23-19-34-40-15-7-6-9-26(23)40/h4-12,15,18-19,21H,13-14,16-17H2,1-3H3,(H,35,43)(H,36,42)(H,37,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) using fluorescent labeled peptide as substrate in presence of ATP at Km concentration by caliper method |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503106
(CHEMBL4537527)Show SMILES COc1ccc2c(cnn2c1)C(=O)Nc1ccc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccc(F)cc23)cc1Cl Show InChI InChI=1S/C33H34ClFN8O4/c1-33(2,3)39-32(46)41-13-11-21(12-14-41)43-28-9-5-19(35)15-23(28)29(40-43)31(45)37-20-6-8-26(25(34)16-20)38-30(44)24-17-36-42-18-22(47-4)7-10-27(24)42/h5-10,15-18,21H,11-14H2,1-4H3,(H,37,45)(H,38,44)(H,39,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503095

(CHEMBL4577049)Show SMILES Cc1cc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccccc23)ccc1NC(=O)c1cnn2ccccc12 Show InChI InChI=1S/C33H36N8O3/c1-21-19-22(12-13-26(21)36-30(42)25-20-34-40-16-8-7-10-27(25)40)35-31(43)29-24-9-5-6-11-28(24)41(38-29)23-14-17-39(18-15-23)32(44)37-33(2,3)4/h5-13,16,19-20,23H,14-15,17-18H2,1-4H3,(H,35,43)(H,36,42)(H,37,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) using fluorescent labeled peptide as substrate in presence of ATP at Km concentration by caliper method |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503102

(CHEMBL4445994)Show SMILES COc1ccc2c(cnn2c1)C(=O)Nc1ccc(NC(=O)c2nn(C3CCN(CC3)C(=O)CC(C)(C)C#N)c3ccc(F)cc23)cc1Cl Show InChI InChI=1S/C34H32ClFN8O4/c1-34(2,19-37)16-30(45)42-12-10-22(11-13-42)44-29-8-4-20(36)14-24(29)31(41-44)33(47)39-21-5-7-27(26(35)15-21)40-32(46)25-17-38-43-18-23(48-3)6-9-28(25)43/h4-9,14-15,17-18,22H,10-13,16H2,1-3H3,(H,39,47)(H,40,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) using fluorescent labeled peptide as substrate in presence of ATP at Km concentration by caliper method |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50305146
(4-amino-5-(2,6-difluorobenzoyl)-2-(4-phenoxyphenyl...)Show SMILES NC(=O)c1c(N)c([nH]c1-c1ccc(Oc2ccccc2)cc1)C(=O)c1c(F)cccc1F Show InChI InChI=1S/C24H17F2N3O3/c25-16-7-4-8-17(26)18(16)23(30)22-20(27)19(24(28)31)21(29-22)13-9-11-15(12-10-13)32-14-5-2-1-3-6-14/h1-12,29H,27H2,(H2,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Csk |
Bioorg Med Chem Lett 20: 108-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.014
BindingDB Entry DOI: 10.7270/Q2Z60P5W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503100

(CHEMBL4443654)Show SMILES CC(C)(C)NC(=O)N1CCC(CC1)n1nc(C(=O)Nc2ccc(NC(=O)c3cnn4ccccc34)c(Cl)c2)c2ccccc12 Show InChI InChI=1S/C32H33ClN8O3/c1-32(2,3)37-31(44)39-16-13-21(14-17-39)41-27-10-5-4-8-22(27)28(38-41)30(43)35-20-11-12-25(24(33)18-20)36-29(42)23-19-34-40-15-7-6-9-26(23)40/h4-12,15,18-19,21H,13-14,16-17H2,1-3H3,(H,35,43)(H,36,42)(H,37,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503106

(CHEMBL4537527)Show SMILES COc1ccc2c(cnn2c1)C(=O)Nc1ccc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccc(F)cc23)cc1Cl Show InChI InChI=1S/C33H34ClFN8O4/c1-33(2,3)39-32(46)41-13-11-21(12-14-41)43-28-9-5-19(35)15-23(28)29(40-43)31(45)37-20-6-8-26(25(34)16-20)38-30(44)24-17-36-42-18-22(47-4)7-10-27(24)42/h5-10,15-18,21H,11-14H2,1-4H3,(H,37,45)(H,38,44)(H,39,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) using fluorescent labeled peptide as substrate in presence of ATP at Km concentration by caliper method |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CSK using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503107
(CHEMBL4437906)Show SMILES CCc1cc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccccc23)ccc1-c1n[nH]c(=O)c(C)c1C Show InChI InChI=1S/C32H39N7O3/c1-7-21-18-22(12-13-24(21)27-19(2)20(3)29(40)36-35-27)33-30(41)28-25-10-8-9-11-26(25)39(37-28)23-14-16-38(17-15-23)31(42)34-32(4,5)6/h8-13,18,23H,7,14-17H2,1-6H3,(H,33,41)(H,34,42)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM93207
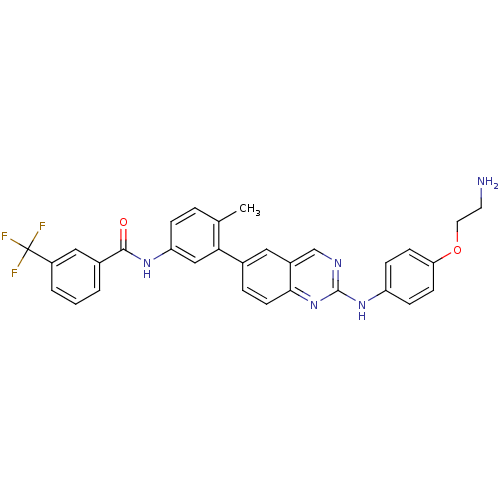
(Kinase inhibitor, 5)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1ccc2nc(Nc3ccc(OCCN)cc3)ncc2c1 Show InChI InChI=1S/C31H26F3N5O2/c1-19-5-7-25(37-29(40)21-3-2-4-23(16-21)31(32,33)34)17-27(19)20-6-12-28-22(15-20)18-36-30(39-28)38-24-8-10-26(11-9-24)41-14-13-35/h2-12,15-18H,13-14,35H2,1H3,(H,37,40)(H,36,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Washington
| Assay Description
Fluorescence assay used for determination of catch and release efficiency. |
ACS Chem Biol 8: 691-9 (2013)
Article DOI: 10.1021/cb300623a
BindingDB Entry DOI: 10.7270/Q20R9N1X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant CSK using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human CSK using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503101

(CHEMBL4469360)Show SMILES COc1ccc2c(cnn2c1)C(=O)Nc1ccc(NC(=O)c2nn(C)c3ccccc23)cc1C Show InChI InChI=1S/C25H22N6O3/c1-15-12-16(27-25(33)23-18-6-4-5-7-21(18)30(2)29-23)8-10-20(15)28-24(32)19-13-26-31-14-17(34-3)9-11-22(19)31/h4-14H,1-3H3,(H,27,33)(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) using fluorescent labeled peptide as substrate in presence of ATP at Km concentration by caliper method |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human CSK using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM81375
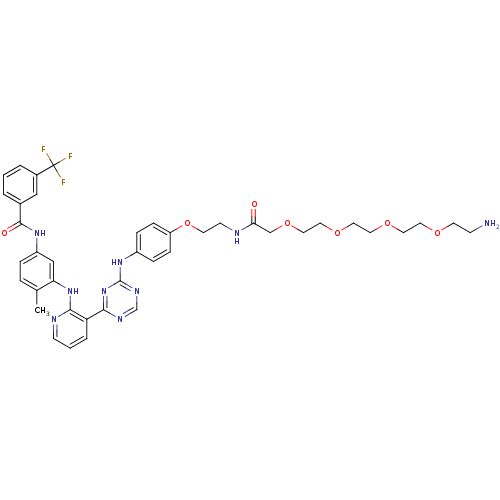
(Amine compound, 12a)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1Nc1ncccc1-c1ncnc(Nc2ccc(OCCNC(=O)COCCOCCOCCOCCN)cc2)n1 Show InChI InChI=1S/C41H46F3N9O7/c1-28-7-8-32(50-39(55)29-4-2-5-30(24-29)41(42,43)44)25-35(28)52-37-34(6-3-14-47-37)38-48-27-49-40(53-38)51-31-9-11-33(12-10-31)60-17-15-46-36(54)26-59-23-22-58-21-20-57-19-18-56-16-13-45/h2-12,14,24-25,27H,13,15-23,26,45H2,1H3,(H,46,54)(H,47,52)(H,50,55)(H,48,49,51,53) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16.3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington
| Assay Description
In vitro activity assay using various kinases. |
Chem Biol 17: 195-206 (2010)
Article DOI: 10.1016/j.chembiol.2010.01.008
BindingDB Entry DOI: 10.7270/Q2833QG7 |
More data for this
Ligand-Target Pair | |
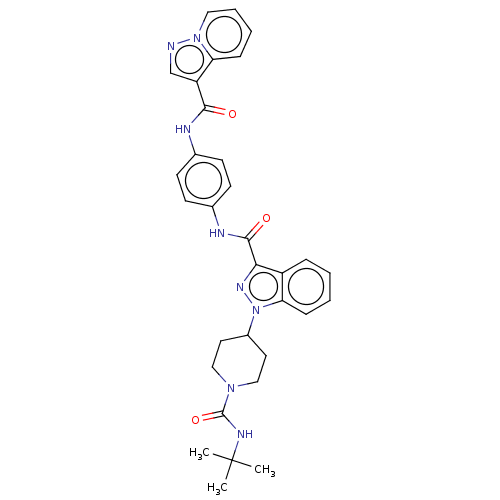
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503105
(CHEMBL4483095)Show SMILES CC(C)(C)NC(=O)N1CCC(CC1)n1nc(C(=O)Nc2ccc(NC(=O)c3cnn4ccccc34)cc2)c2ccccc12 Show InChI InChI=1S/C32H34N8O3/c1-32(2,3)36-31(43)38-18-15-23(16-19-38)40-27-10-5-4-8-24(27)28(37-40)30(42)35-22-13-11-21(12-14-22)34-29(41)25-20-33-39-17-7-6-9-26(25)39/h4-14,17,20,23H,15-16,18-19H2,1-3H3,(H,34,41)(H,35,42)(H,36,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) using fluorescent labeled peptide as substrate in presence of ATP at Km concentration by caliper method |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503105

(CHEMBL4483095)Show SMILES CC(C)(C)NC(=O)N1CCC(CC1)n1nc(C(=O)Nc2ccc(NC(=O)c3cnn4ccccc34)cc2)c2ccccc12 Show InChI InChI=1S/C32H34N8O3/c1-32(2,3)36-31(43)38-18-15-23(16-19-38)40-27-10-5-4-8-24(27)28(37-40)30(42)35-22-13-11-21(12-14-22)34-29(41)25-20-33-39-17-7-6-9-26(25)39/h4-14,17,20,23H,15-16,18-19H2,1-3H3,(H,34,41)(H,35,42)(H,36,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
HANGZHOU HERTZ PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Preparation of Compounds to be Tested:1) Using DMSO to prepare 50× compound stock solutions (same as the stock solution in Example 34) for later use;... |
US Patent US10711006 (2020)
BindingDB Entry DOI: 10.7270/Q2WH2T10 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50114585

((R)-7-[4-(4-Methyl-piperazin-1-yl)-cyclohexyl]-5-(...)Show SMILES CN1CCN(CC1)[C@H]1CC[C@H](CC1)n1cc(-c2ccc(Oc3ccccc3)cc2)c2c(N)ncnc12 |wU:7.7,10.14,(2.19,-10.22,;1.73,-8.75,;2.75,-7.59,;2.29,-6.14,;.77,-5.83,;-.26,-6.95,;.21,-8.41,;.3,-4.36,;-1.21,-4.04,;-1.67,-2.57,;-.63,-1.42,;.86,-1.75,;1.33,-3.22,;-1.1,.05,;-.19,1.28,;-1.1,2.52,;-.61,4.01,;-1.63,5.13,;-1.16,6.58,;.35,6.92,;.84,8.36,;2.34,8.68,;2.81,10.15,;4.32,10.44,;5.35,9.29,;4.88,7.84,;3.36,7.52,;1.38,5.76,;.89,4.29,;-2.56,2.05,;-3.89,2.82,;-3.89,4.36,;-5.22,2.05,;-5.23,.54,;-3.9,-.26,;-2.57,.51,)| Show InChI InChI=1S/C29H34N6O/c1-33-15-17-34(18-16-33)22-9-11-23(12-10-22)35-19-26(27-28(30)31-20-32-29(27)35)21-7-13-25(14-8-21)36-24-5-3-2-4-6-24/h2-8,13-14,19-20,22-23H,9-12,15-18H2,1H3,(H2,30,31,32)/t22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human Tyrosine-protein kinase CSK |
Bioorg Med Chem Lett 12: 1683-6 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZGR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503101

(CHEMBL4469360)Show SMILES COc1ccc2c(cnn2c1)C(=O)Nc1ccc(NC(=O)c2nn(C)c3ccccc23)cc1C Show InChI InChI=1S/C25H22N6O3/c1-15-12-16(27-25(33)23-18-6-4-5-7-21(18)30(2)29-23)8-10-20(15)28-24(32)19-13-26-31-14-17(34-3)9-11-22(19)31/h4-14H,1-3H3,(H,27,33)(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
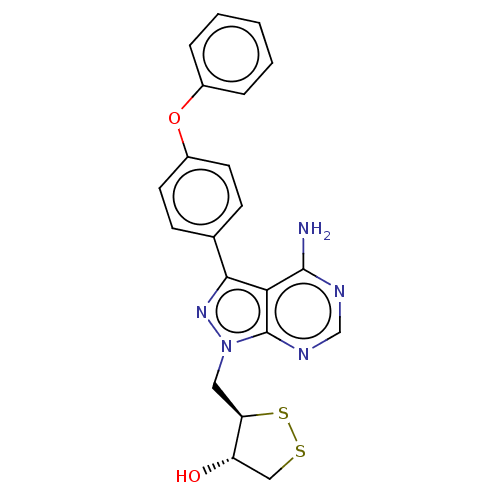
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM473171
((3R,4S)-3-((4-amino-3-(4-phenoxyphenyl)-1H-pyrazol...)Show SMILES Nc1ncnc2n(C[C@H]3SSC[C@@H]3O)nc(-c3ccc(Oc4ccccc4)cc3)c12 |r| Show InChI InChI=1S/C21H19N5O2S2/c22-20-18-19(13-6-8-15(9-7-13)28-14-4-2-1-3-5-14)25-26(21(18)24-12-23-20)10-17-16(27)11-29-30-17/h1-9,12,16-17,27H,10-11H2,(H2,22,23,24)/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
SABILA BIOSCIENCES LLC
US Patent
| Assay Description
IC50 Table 13-20: The protocol calls for test compound of the invention to be incubated with kinase, substrate, cofactors, and radio-isotope-labeled ... |
US Patent US10844038 (2020)
BindingDB Entry DOI: 10.7270/Q25D8VZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CSK |
Leukemia 23: 477-85 (2009)
Article DOI: 10.1038/leu.2008.334
BindingDB Entry DOI: 10.7270/Q22Z15R6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503104

(CHEMBL4591137)Show SMILES CCc1cc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccccc23)ccc1-c1n[nH]c(=O)cc1C Show InChI InChI=1S/C31H37N7O3/c1-6-20-18-21(11-12-23(20)27-19(2)17-26(39)34-35-27)32-29(40)28-24-9-7-8-10-25(24)38(36-28)22-13-15-37(16-14-22)30(41)33-31(3,4)5/h7-12,17-18,22H,6,13-16H2,1-5H3,(H,32,40)(H,33,41)(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503103
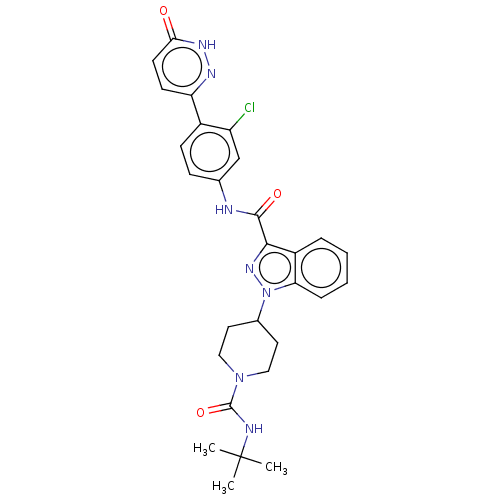
(CHEMBL4555519)Show SMILES CC(C)(C)NC(=O)N1CCC(CC1)n1nc(C(=O)Nc2ccc(c(Cl)c2)-c2ccc(=O)[nH]n2)c2ccccc12 Show InChI InChI=1S/C28H30ClN7O3/c1-28(2,3)31-27(39)35-14-12-18(13-15-35)36-23-7-5-4-6-20(23)25(34-36)26(38)30-17-8-9-19(21(29)16-17)22-10-11-24(37)33-32-22/h4-11,16,18H,12-15H2,1-3H3,(H,30,38)(H,31,39)(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50340571
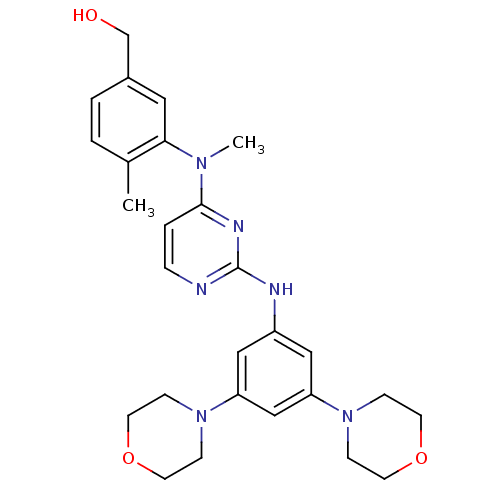
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C27H34N6O3/c1-20-3-4-21(19-34)15-25(20)31(2)26-5-6-28-27(30-26)29-22-16-23(32-7-11-35-12-8-32)18-24(17-22)33-9-13-36-14-10-33/h3-6,15-18,34H,7-14,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of CSK |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50503108
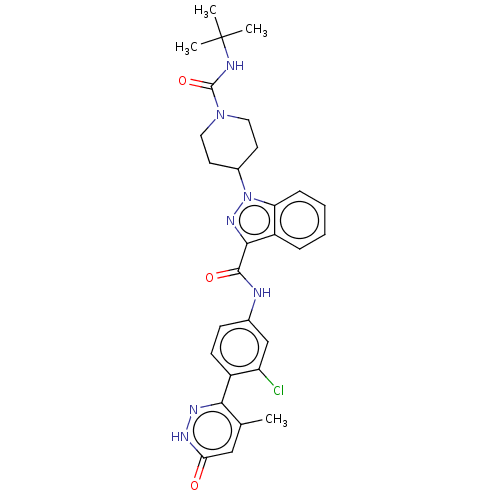
(CHEMBL4518970)Show SMILES Cc1cc(=O)[nH]nc1-c1ccc(NC(=O)c2nn(C3CCN(CC3)C(=O)NC(C)(C)C)c3ccccc23)cc1Cl Show InChI InChI=1S/C29H32ClN7O3/c1-17-15-24(38)33-34-25(17)20-10-9-18(16-22(20)30)31-27(39)26-21-7-5-6-8-23(21)37(35-26)19-11-13-36(14-12-19)28(40)32-29(2,3)4/h5-10,15-16,19H,11-14H2,1-4H3,(H,31,39)(H,32,40)(H,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged/GST-tagged CSK (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 10: 1486-1491 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00354
BindingDB Entry DOI: 10.7270/Q25142G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM81376
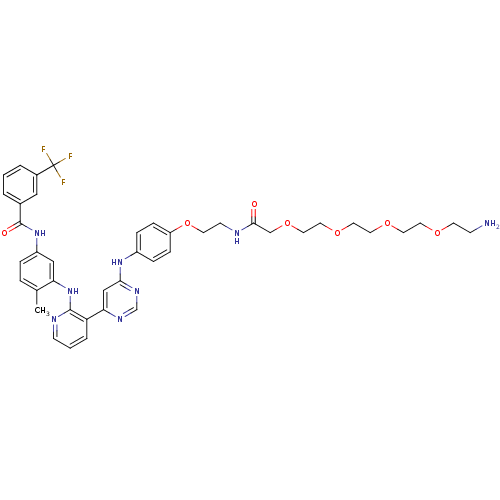
(Amine compound, 13a)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1Nc1ncccc1-c1cc(Nc2ccc(OCCNC(=O)COCCOCCOCCOCCN)cc2)ncn1 Show InChI InChI=1S/C42H47F3N8O7/c1-29-7-8-33(52-41(55)30-4-2-5-31(24-30)42(43,44)45)25-36(29)53-40-35(6-3-14-48-40)37-26-38(50-28-49-37)51-32-9-11-34(12-10-32)60-17-15-47-39(54)27-59-23-22-58-21-20-57-19-18-56-16-13-46/h2-12,14,24-26,28H,13,15-23,27,46H2,1H3,(H,47,54)(H,48,53)(H,52,55)(H,49,50,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington
| Assay Description
In vitro activity assay using various kinases. |
Chem Biol 17: 195-206 (2010)
Article DOI: 10.1016/j.chembiol.2010.01.008
BindingDB Entry DOI: 10.7270/Q2833QG7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM255256
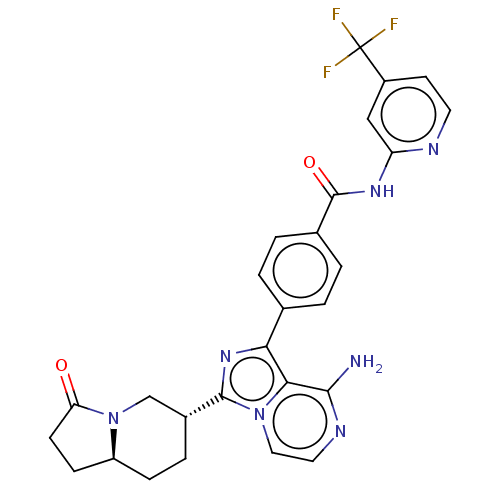
(US9481682, 4)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CCC(=O)N2C1 |r| Show InChI InChI=1S/C27H24F3N7O2/c28-27(29,30)18-9-10-32-20(13-18)34-26(39)16-3-1-15(2-4-16)22-23-24(31)33-11-12-36(23)25(35-22)17-5-6-19-7-8-21(38)37(19)14-17/h1-4,9-13,17,19H,5-8,14H2,(H2,31,33)(H,32,34,39)/t17-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CSK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127390
BindingDB Entry DOI: 10.7270/Q22N55Z9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM452963

(US10711006, Compound I)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)nc3)c12)C1CCCN(C1)C(=O)C(Br)=C Show InChI InChI=1S/C24H22BrN7O2/c1-15(25)24(33)31-11-5-6-17(13-31)32-23-20(22(26)28-14-29-23)21(30-32)16-9-10-19(27-12-16)34-18-7-3-2-4-8-18/h2-4,7-10,12,14,17H,1,5-6,11,13H2,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
HANGZHOU HERTZ PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Preparation of Compounds to be Tested:1) Using DMSO to prepare 50× compound stock solutions (same as the stock solution in Example 34) for later use;... |
US Patent US10711006 (2020)
BindingDB Entry DOI: 10.7270/Q2WH2T10 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50577406
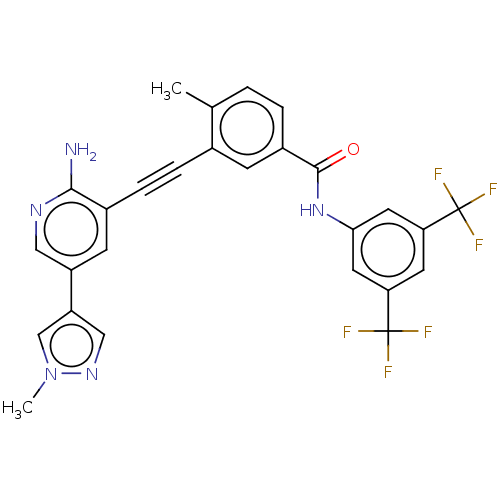
(CHEMBL4862806)Show SMILES Cc1ccc(cc1C#Cc1cc(cnc1N)-c1cnn(C)c1)C(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant CSK by radiometric scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00976
BindingDB Entry DOI: 10.7270/Q2J96B6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM267790
(4-(8-amino-3-{(3R,6S)-6-methyl-1-[(3-methyloxetan-...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C1CC1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C29H27F4N7O2/c1-15-2-3-18(14-40(15)28(42)16-4-5-16)26-38-23(24-25(34)36-10-11-39(24)26)20-7-6-17(12-21(20)30)27(41)37-22-13-19(8-9-35-22)29(31,32)33/h6-13,15-16,18H,2-5,14H2,1H3,(H2,34,36)(H,35,37,41)/t15-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CSK |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.5b00463
BindingDB Entry DOI: 10.7270/Q25B065B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM255338

(US9481682, 116 | US9481682, 88)Show SMILES COc1cc(ccc1-c1nc([C@@H]2CC[C@H]3CCC(=O)N3C2)n2ccnc(N)c12)C(=O)Nc1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C28H26F3N7O3/c1-41-20-12-15(27(40)35-21-13-17(8-9-33-21)28(29,30)31)3-6-19(20)23-24-25(32)34-10-11-37(24)26(36-23)16-2-4-18-5-7-22(39)38(18)14-16/h3,6,8-13,16,18H,2,4-5,7,14H2,1H3,(H2,32,34)(H,33,35,40)/t16-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CSK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127390
BindingDB Entry DOI: 10.7270/Q22N55Z9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50558112
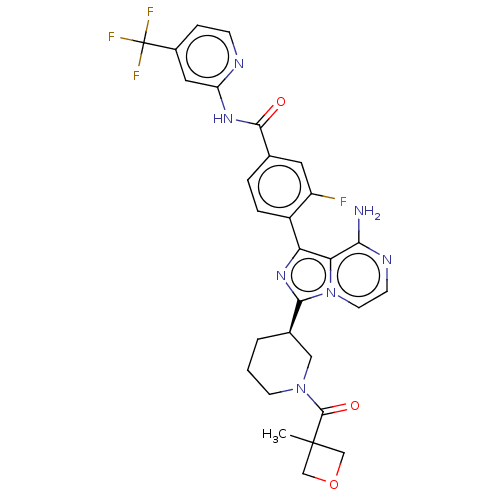
(CHEMBL4799627)Show SMILES CC1(COC1)C(=O)N1CCC[C@H](C1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CSK |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.5b00463
BindingDB Entry DOI: 10.7270/Q25B065B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
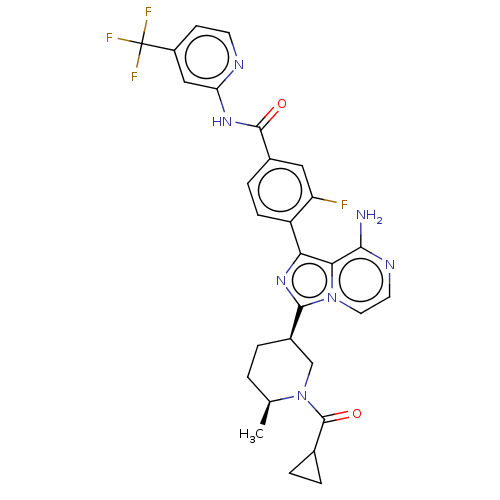
(Homo sapiens (Human)) | BDBM267959
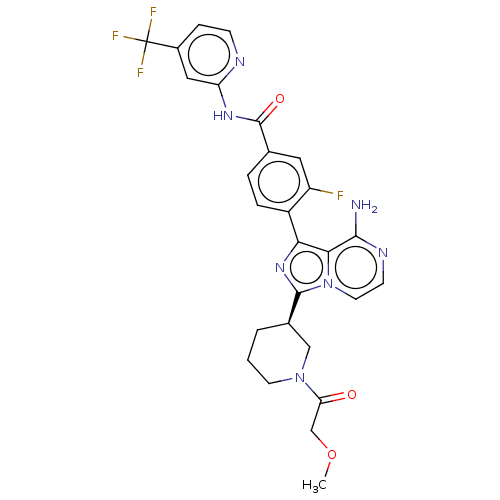
(4-(8-amino-3-{(3R)-1-[(3-methyloxetan-3-yl)carbony...)Show SMILES COCC(=O)N1CCC[C@H](C1)c1nc(-c2ccc(cc2F)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| Show InChI InChI=1S/C27H25F4N7O3/c1-41-14-21(39)37-9-2-3-16(13-37)25-36-22(23-24(32)34-8-10-38(23)25)18-5-4-15(11-19(18)28)26(40)35-20-12-17(6-7-33-20)27(29,30)31/h4-8,10-12,16H,2-3,9,13-14H2,1H3,(H2,32,34)(H,33,35,40)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Early Development and Discovery Sciences, MRL, Merck& Co., Inc., 126 East Lincoln Avenue, Rahway, NJ 07065, USA. Electronic address: sobhana.babu.boga@merck.com.
Curated by ChEMBL
| Assay Description
Inhibition of CSK (unknown origin) |
Bioorg Med Chem Lett 27: 3939-3943 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.040
BindingDB Entry DOI: 10.7270/Q2959M2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50096279
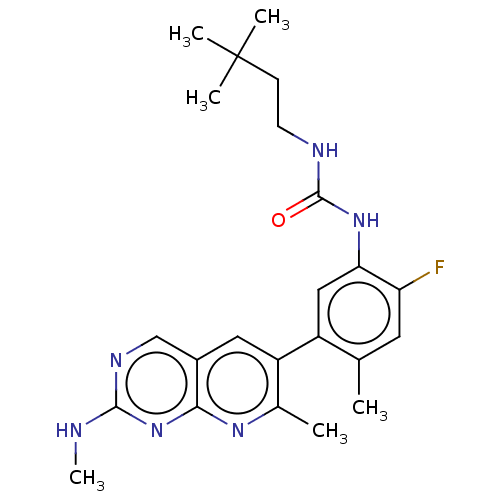
(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive binding affinity to CSK in human A375 cells after 15 mins in presence of ATP analogue |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data