

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50615643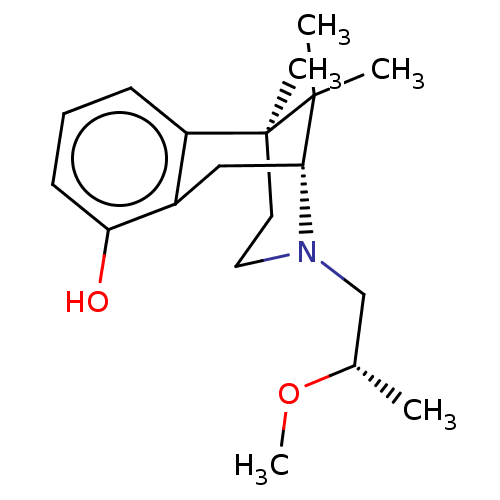 (CHEMBL5286162) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50615644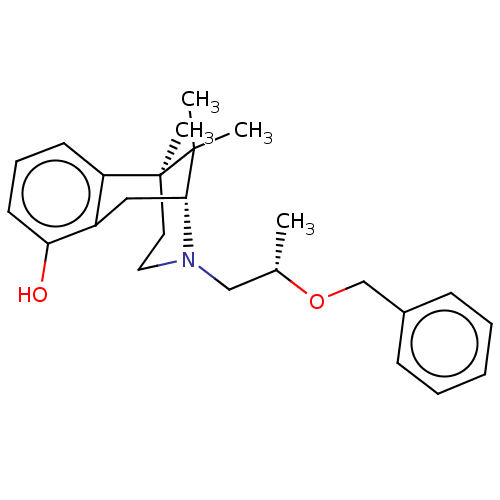 (CROBENETINE | Crobenetine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 322 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208436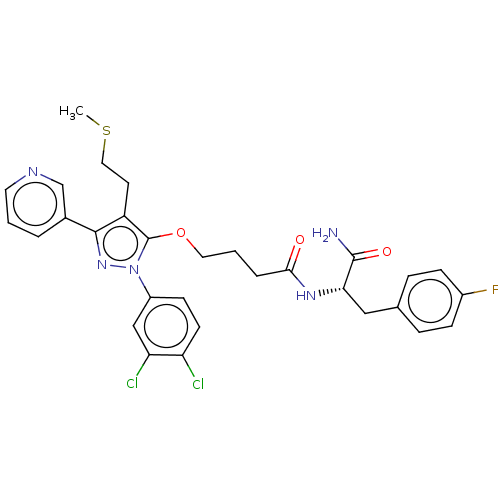 (CHEMBL3885040) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208427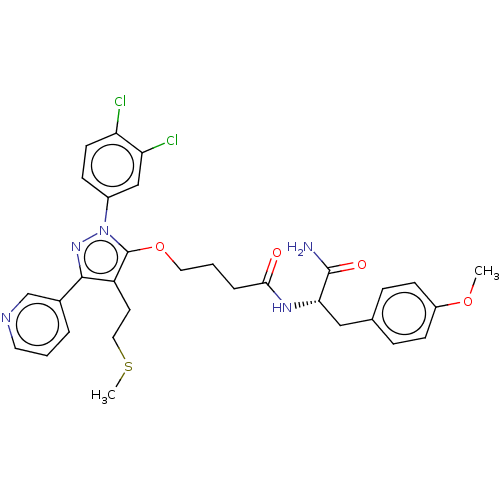 (CHEMBL3884872) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM14713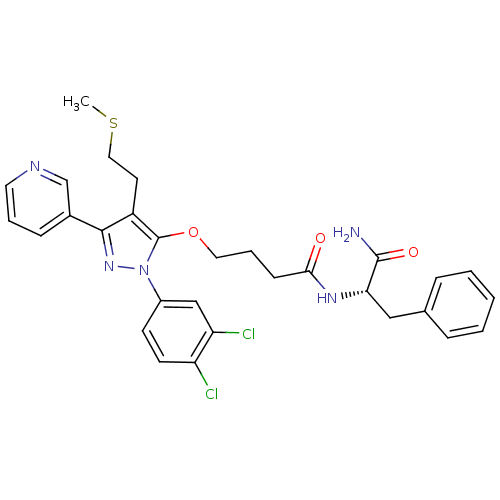 (GGTI-DU40 | N-[(1S)-1-carbamoyl-2-phenylethyl]-4-{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208433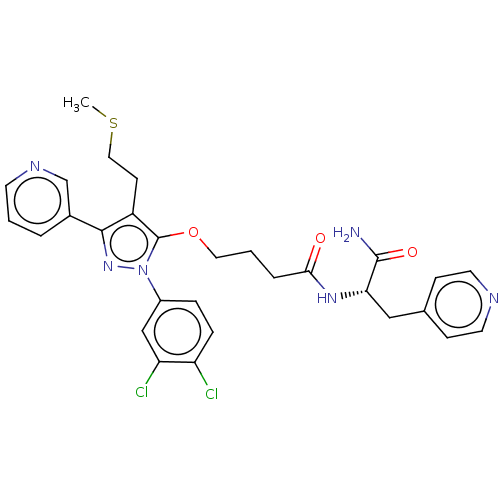 (CHEMBL3885443) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208426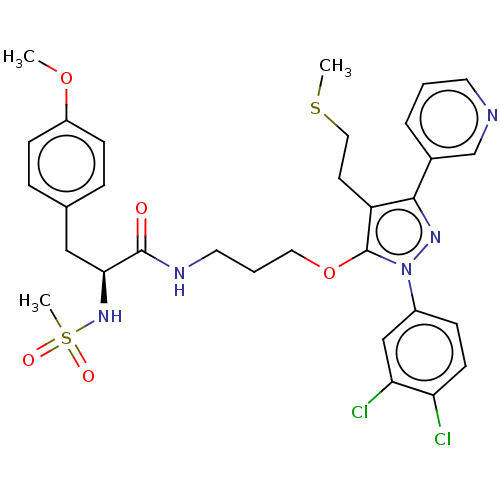 (CHEMBL3883795) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208438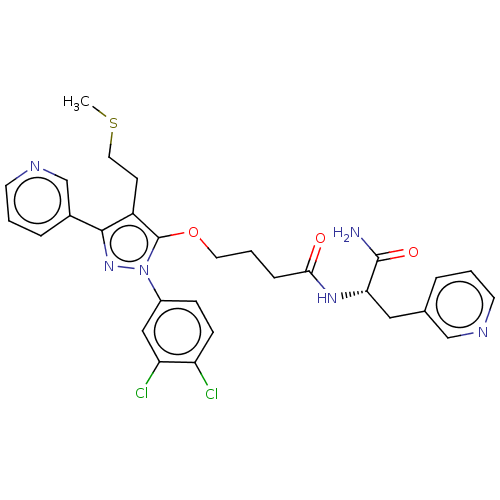 (CHEMBL3885188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248738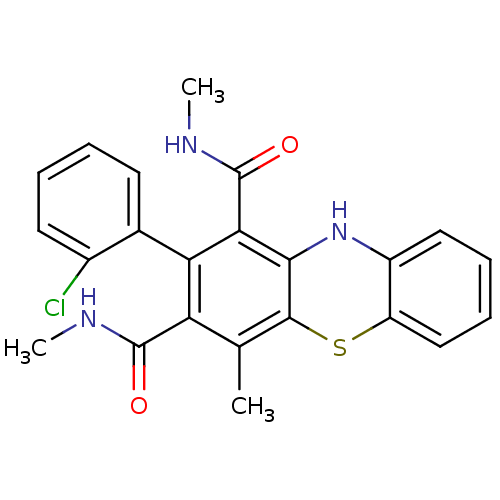 (2-(2-Chlorophenyl)-N,N',4-trimethyl-10Hphenothiazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248772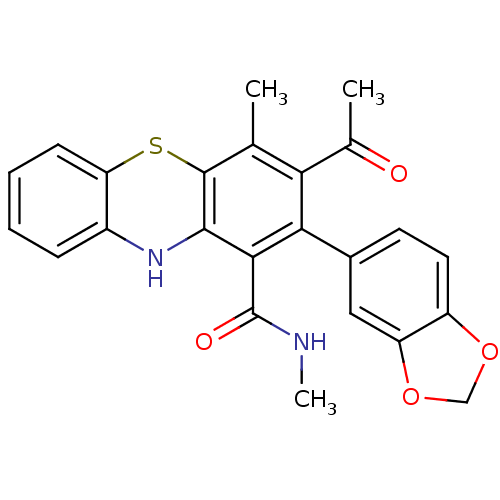 (2-(1,3-benzodioxol-5-yl)-N,N',4-trimethyl-10Hpheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248626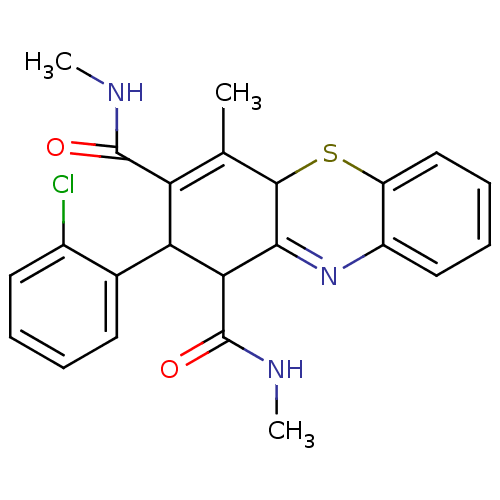 (2-(2-Chlorophenyl)-N,N',4-trimethyl-2,10-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208434 (CHEMBL3884826) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208431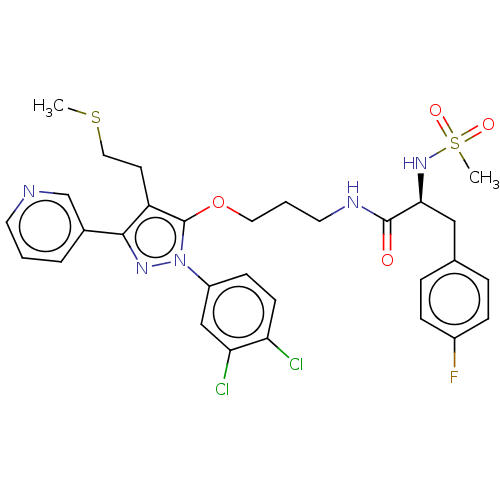 (CHEMBL3883522) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248739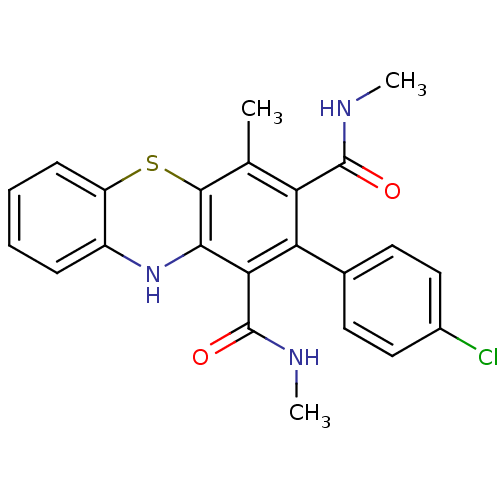 (2-(4-Chlorophenyl)-N,N',4-trimethyl-10Hphenothiazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248736 (2-Phenyl-N,N',4-trimethyl-10H-phenothiazine-1,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248624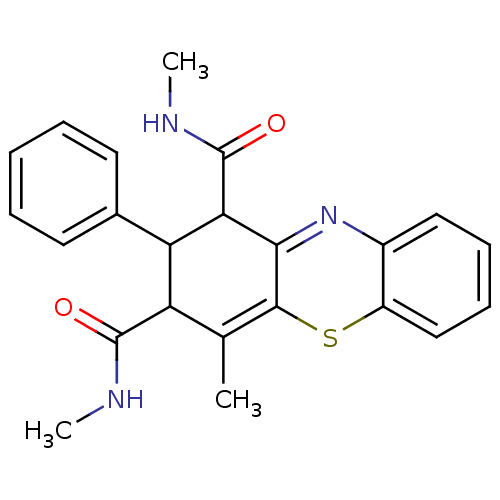 (2-Phenyl-N,N',4-trimethyl-2,10-dihydro-1Hphenothia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248771 (2-(4-Methoxyphenyl)-N,N',4-trimethyl-10Hphenothiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248773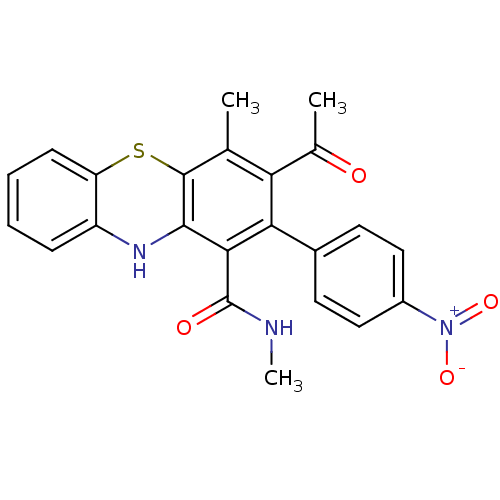 (2-(4-Nitrophenyl)-N,N',4-trimethyl-10Hphenothiazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248625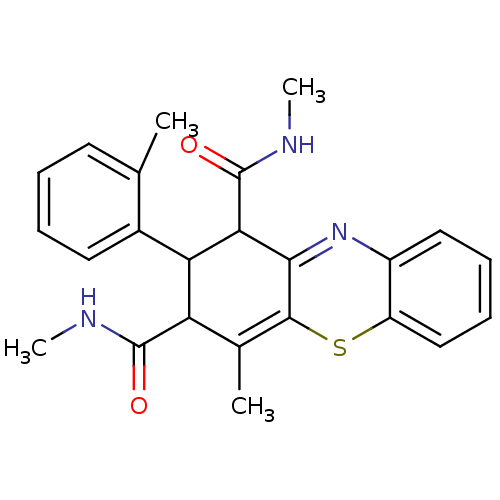 (2-(2-Methylphenyl)-N,N',4-trimethyl-2,10-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208423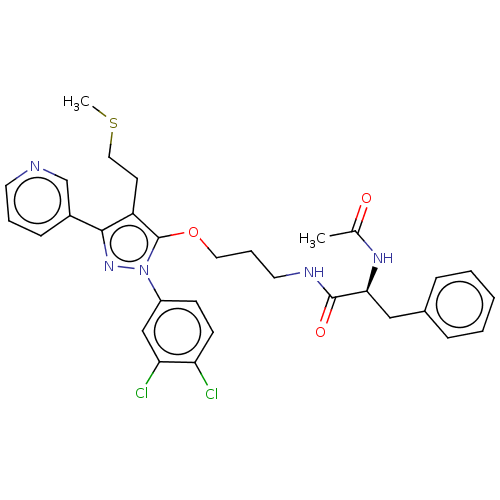 (CHEMBL3884658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248701 (2-(4-Methoxyphenyl)-N,N',4-trimethyl-2,10-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248737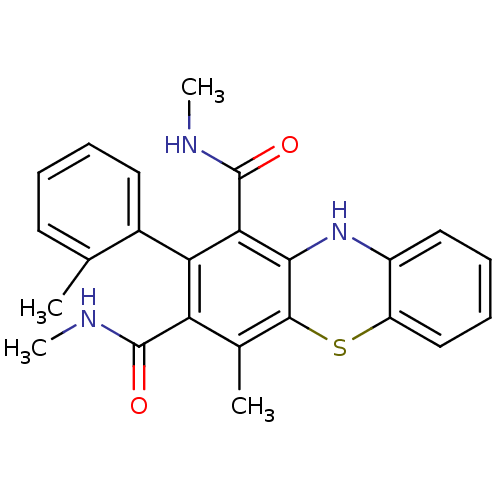 (2-(2-Methylphenyl)-N,N',4-trimethyl-10Hphenothiazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208437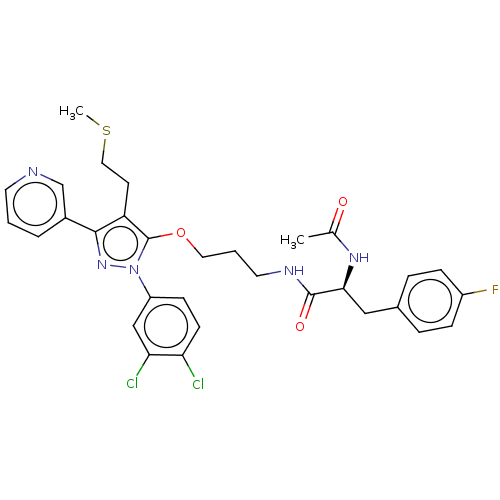 (CHEMBL3884852) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208430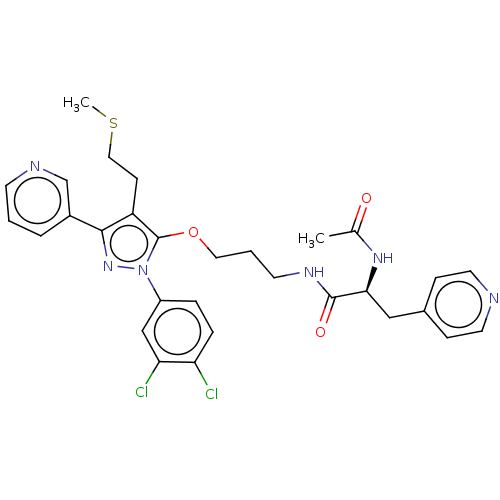 (CHEMBL3884083) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248770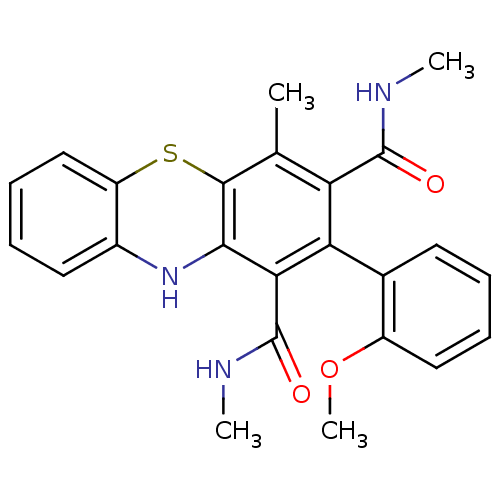 (2-(2-Methoxyphenyl)-N,N',4-trimethyl-10Hphenothiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248700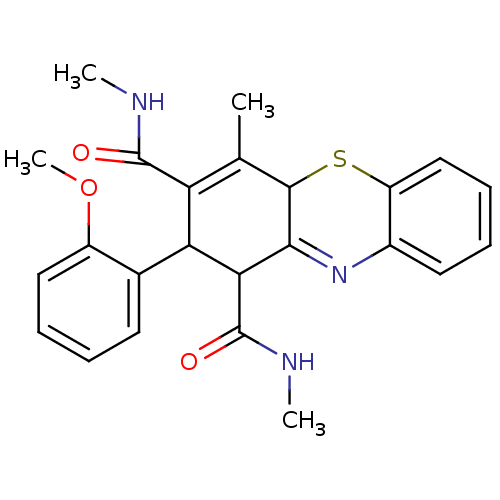 (2-(2-Methoxyphenyl)-N,N',4-trimethyl-2,10-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248702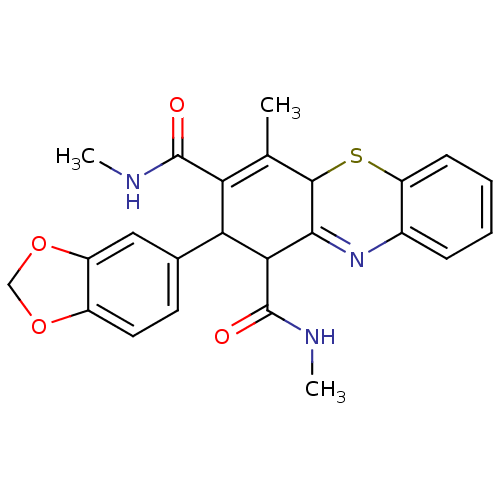 (2-(1,3-Benzodioxol-5-yl)-N,N',4-trimethyl-2,10-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208429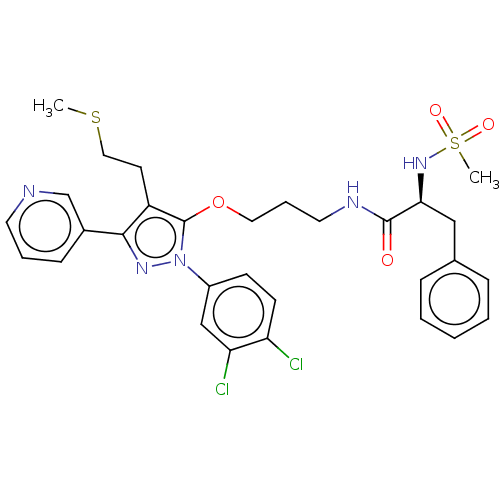 (CHEMBL3883400) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208435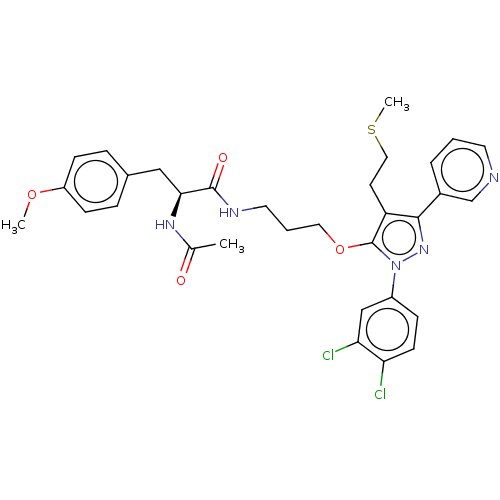 (CHEMBL3885260) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248655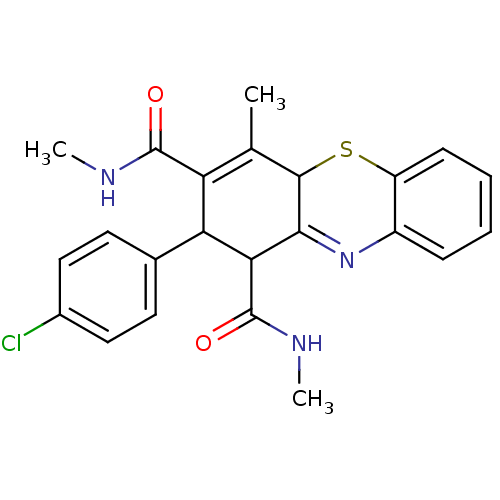 (2-(4-Chlorophenyl)-N,N',4-trimethyl-2,10-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM22360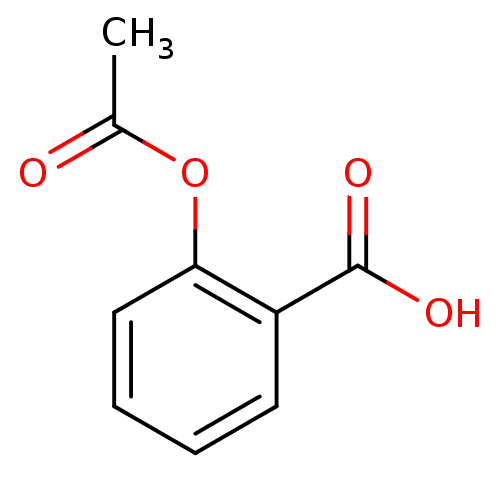 (2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50248703 (2-(4-Nitrophenyl)-N,N',4-trimethyl-2,10-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of gamma-glutamyltranspeptidase (unknown origin) by colorimetric assay | Eur J Med Chem 44: 197-202 (2008) Article DOI: 10.1016/j.ejmech.2008.02.028 BindingDB Entry DOI: 10.7270/Q20K28B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208425 (CHEMBL3884603) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208428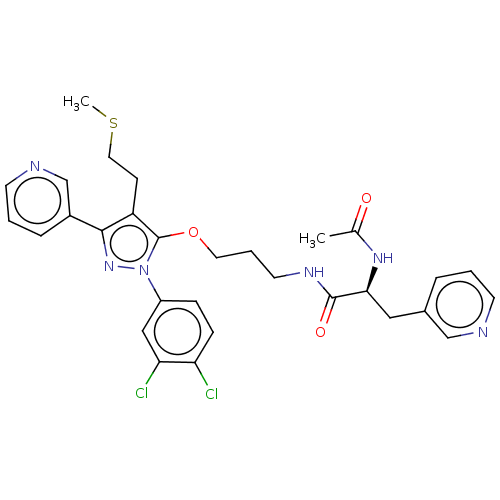 (CHEMBL3883981) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione hydrolase 1 proenzyme (Homo sapiens (Human)) | BDBM50208432 (CHEMBL3884346) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Fahd University of Petroleum and Minerals Curated by ChEMBL | Assay Description Inhibition of recombinant GGT1 (unknown origin) using GGPP as substrate preincubated for 5 mins followed by protein addition measured over 20 mins by... | Eur J Med Chem 124: 666-676 (2016) Article DOI: 10.1016/j.ejmech.2016.09.002 BindingDB Entry DOI: 10.7270/Q2XG9T3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||