

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375312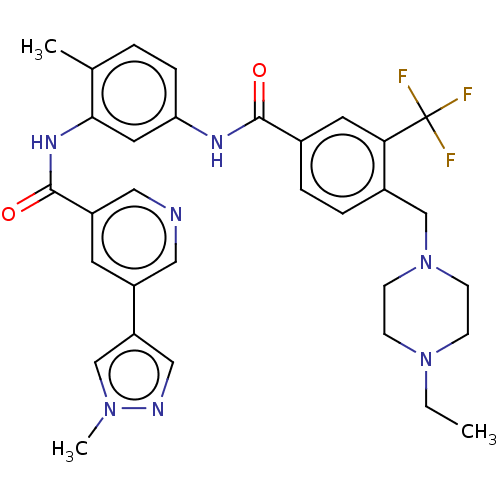 (US9908872, Compound (I-9)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.43 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375310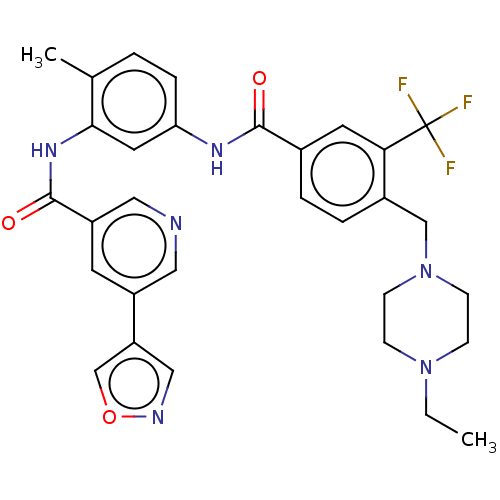 (US9908872, Compound (I-8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.33 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375276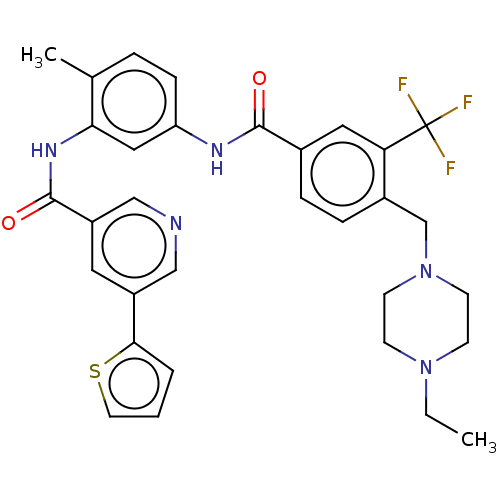 (US10597387, Compound (I-1) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50379186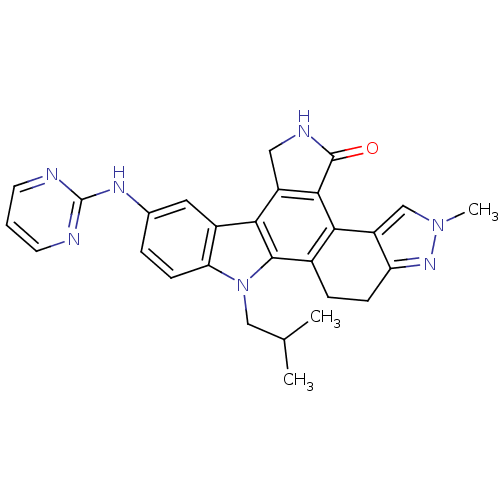 (CEP-11981 | CHEMBL2010872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Inhibition of human TAK1 using ATP as substrate | J Med Chem 55: 903-13 (2012) Article DOI: 10.1021/jm201449n BindingDB Entry DOI: 10.7270/Q2ZS2XHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375281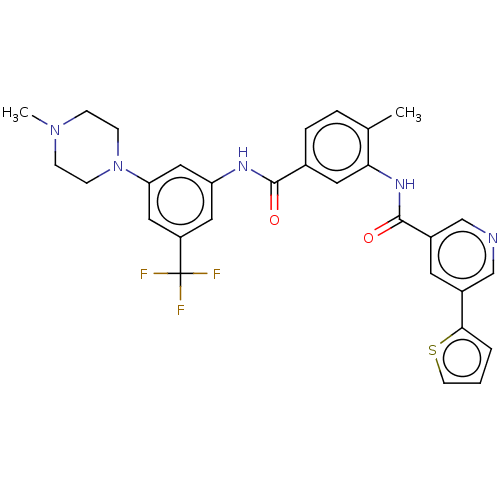 (US10597387, Compound (I-4) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375307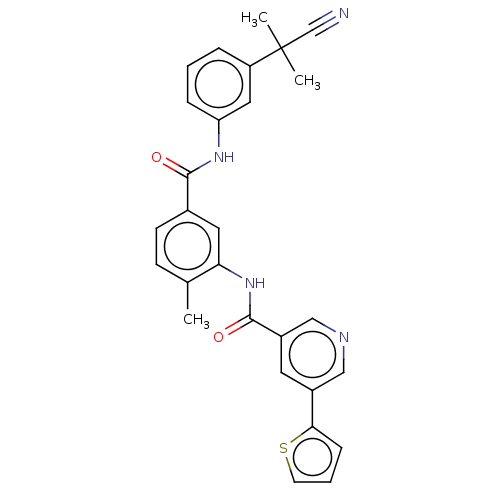 (US10597387, Compound (I-6) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29.9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439303 (US10633348, Compound (A-17)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439318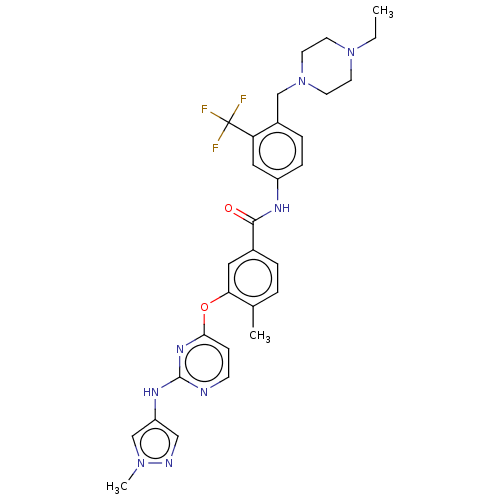 (US10633348, Compound (A-14)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375308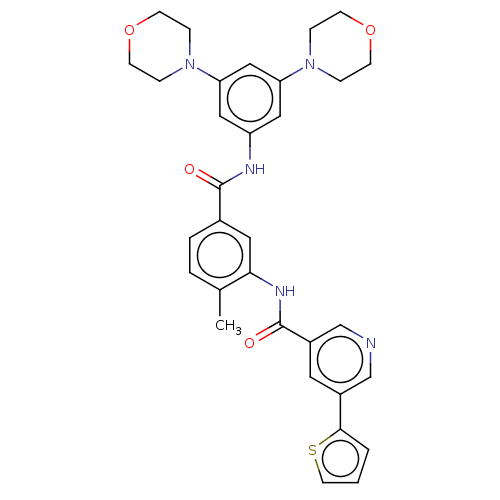 (US10597387, Compound (I-7) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 56.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50086441 (CHEMBL3426225 | US10266537, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human TAK1 | J Med Chem 58: 3957-74 (2015) Article DOI: 10.1021/acs.jmedchem.5b00270 BindingDB Entry DOI: 10.7270/Q2J38V91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375280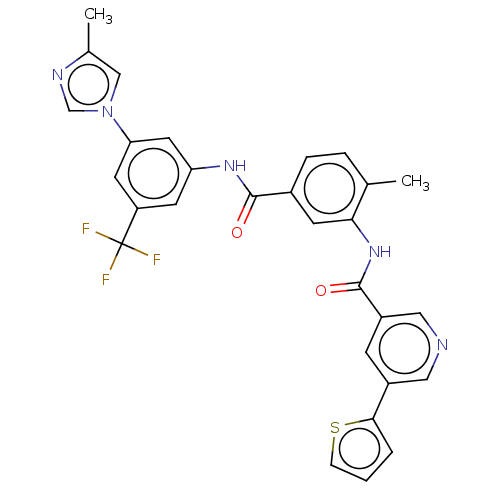 (US10597387, Compound (I-3) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 62.2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439308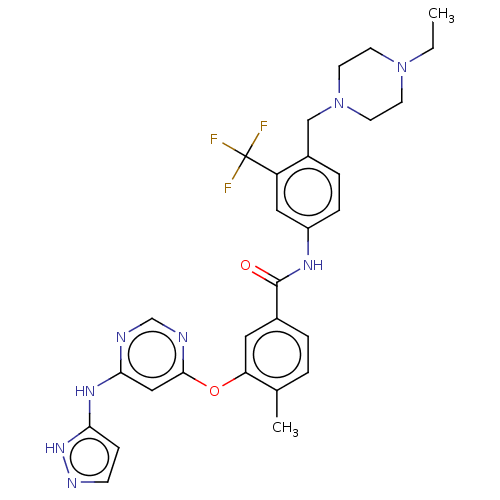 (US10633348, Compound (A-6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439309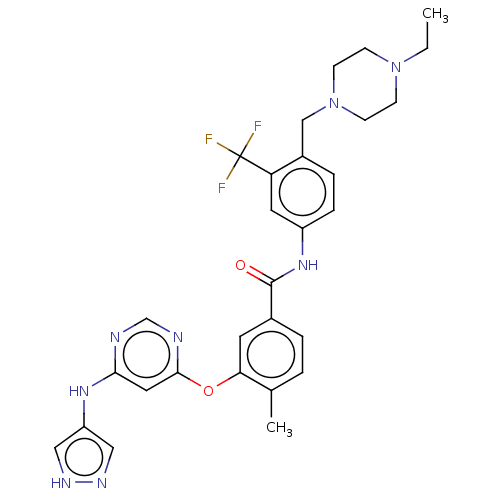 (US10633348, Compound (A-7)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439316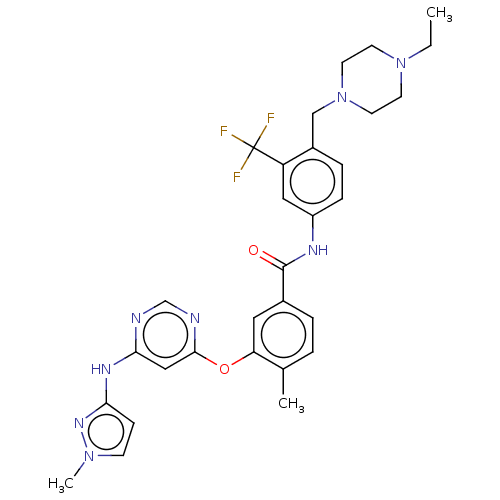 (US10633348, Compound (A-12)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439305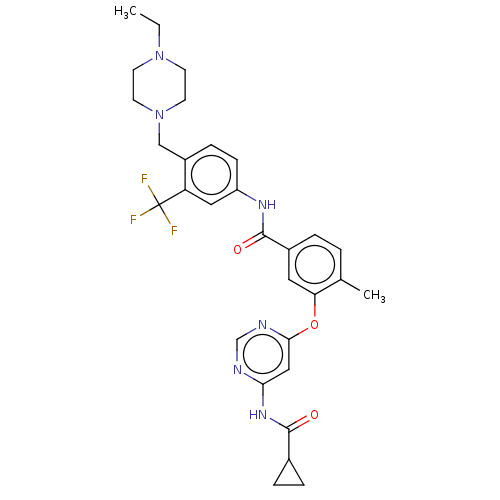 (US10633348, Compound (A-3)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439307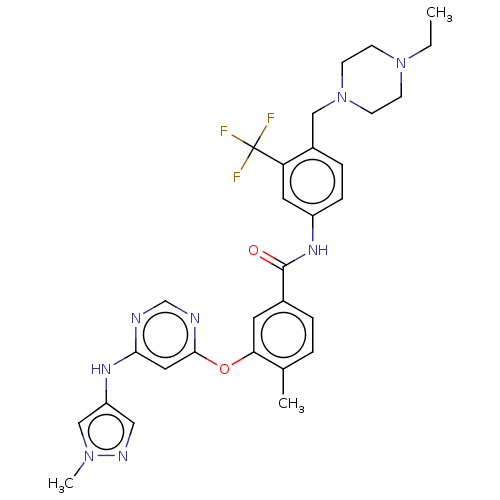 (US10633348, Compound (A-5)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439304 (US10633348, Compound (A-2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375299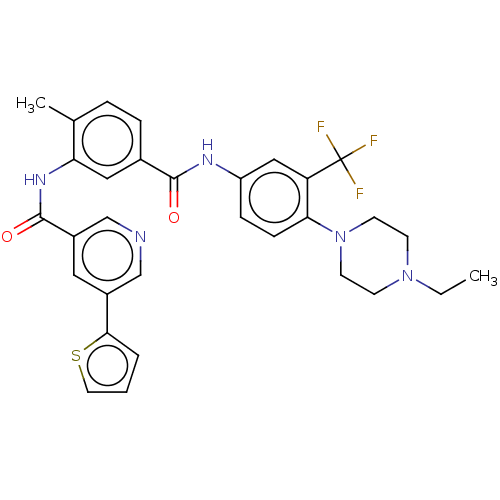 (US10597387, Compound (I-5) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2668GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50604141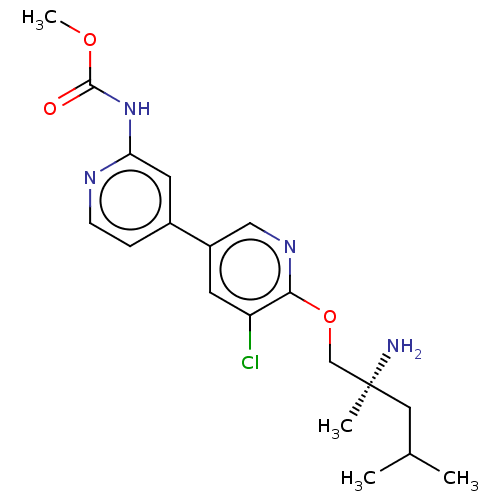 (CHEMBL5188433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02132 BindingDB Entry DOI: 10.7270/Q2TH8RSP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50584720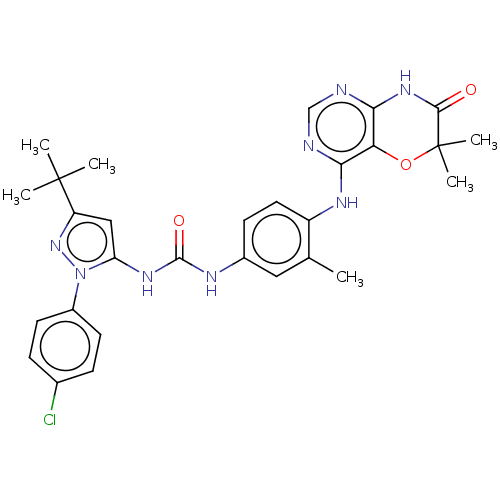 (CHEMBL5088153) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TAK1 in presence of ATP by radiometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01597 BindingDB Entry DOI: 10.7270/Q2377DMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439319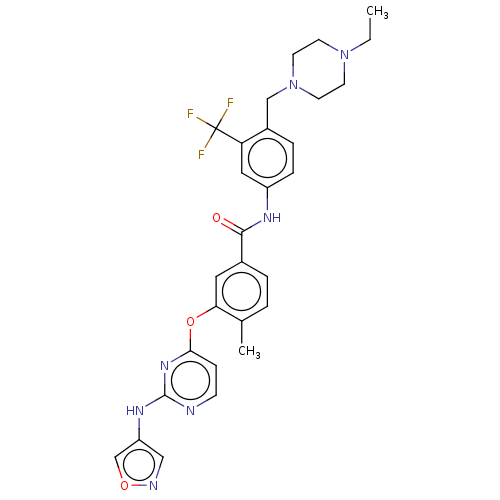 (US10633348, Compound (A-15)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50604140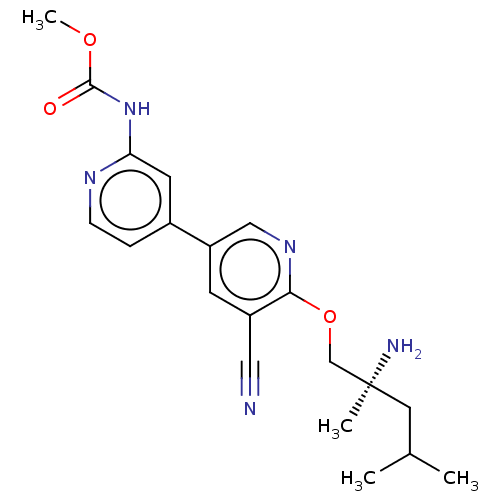 (CHEMBL5185000) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02132 BindingDB Entry DOI: 10.7270/Q2TH8RSP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM438001 (US10633348, Compound (A-1) | US10633348, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439317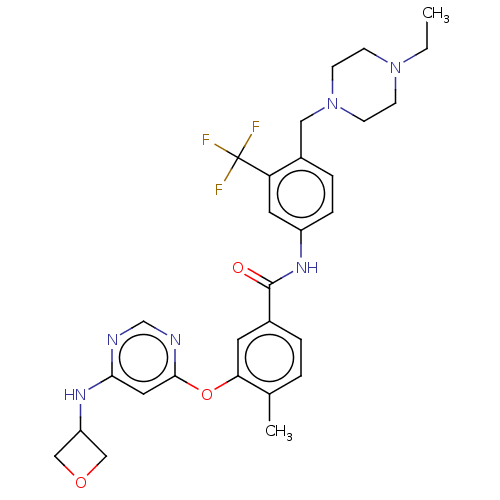 (US10633348, Compound (A-13)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM438001 (US10633348, Compound (A-1) | US10633348, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 487 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439306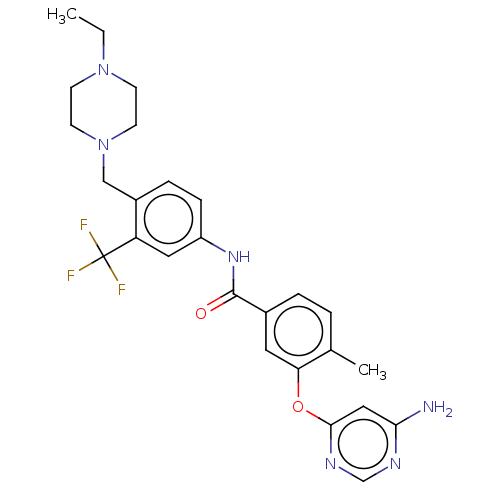 (US10633348, Compound (A-4)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 591 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM400813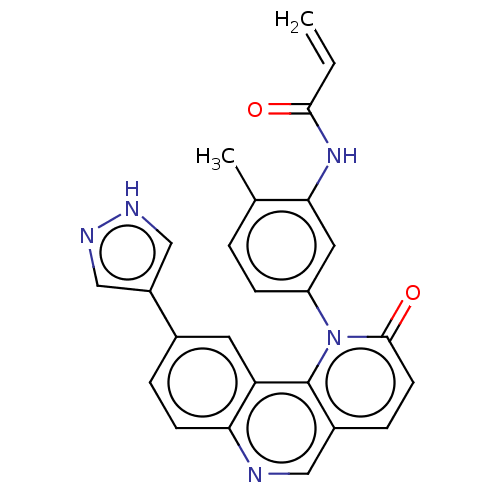 (QL-X-138 | US10000483, Compound II-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 717 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech... | J Med Chem 52: 6621-36 (2009) BindingDB Entry DOI: 10.7270/Q2QF8W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439314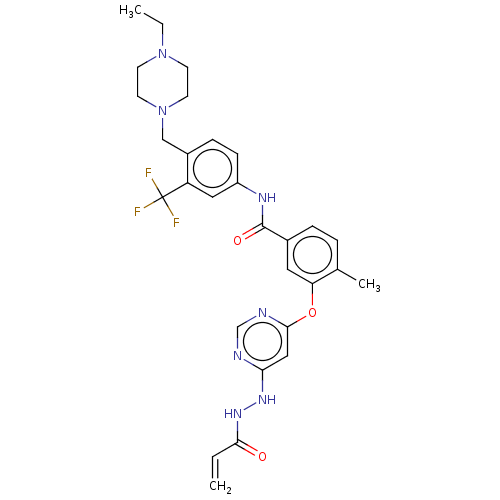 (US10633348, Compound (A-18)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50245587 (CHEMBL4077064 | US10000483, Compound II-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech... | J Med Chem 52: 6621-36 (2009) BindingDB Entry DOI: 10.7270/Q2QF8W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM102620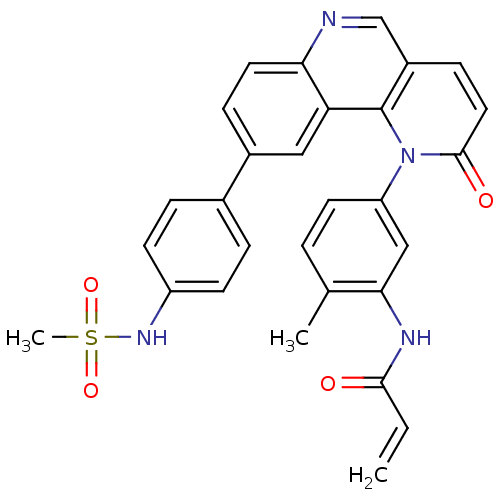 (BMX-IN-1 | N-[5-[9-[4-(methanesulfonamido)phenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech... | J Med Chem 52: 6621-36 (2009) BindingDB Entry DOI: 10.7270/Q2QF8W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375307 (US10597387, Compound (I-6) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439312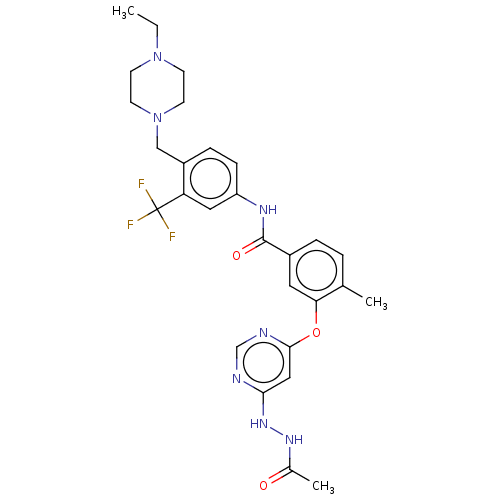 (US10633348, Compound (A-9)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375276 (US10597387, Compound (I-1) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439315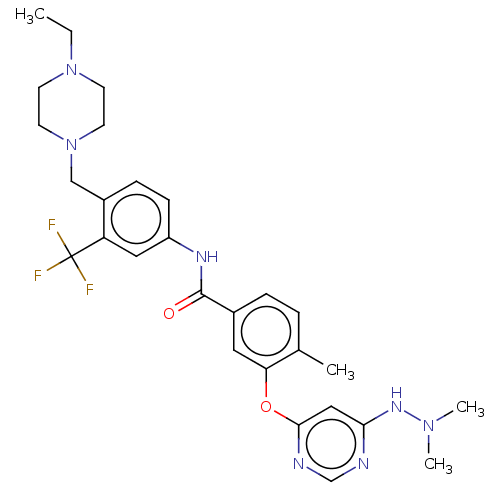 (US10633348, Compound (A-11)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM439313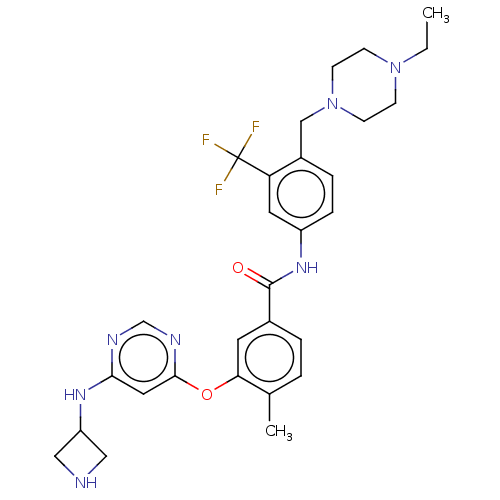 (US10633348, Compound (A-10)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ... | US Patent US10633348 (2020) BindingDB Entry DOI: 10.7270/Q22Z18JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM375299 (US10597387, Compound (I-5) | US9908872, Compound (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50386693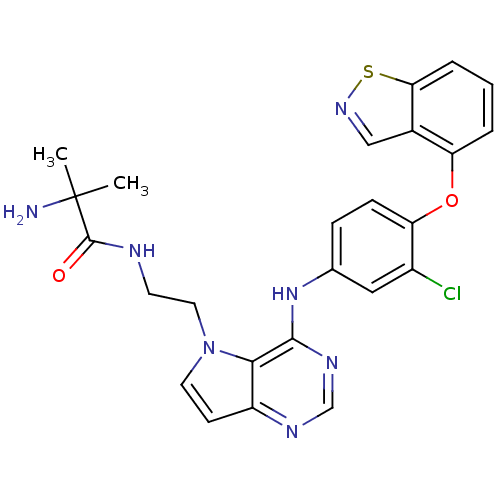 (CHEMBL2048912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminus FLAG-tagged TAK1 expressed in baculovirus expression system using [gamma-33P]-ATP measured after 60 mins by scintillation co... | J Med Chem 55: 3975-91 (2012) Article DOI: 10.1021/jm300185p BindingDB Entry DOI: 10.7270/Q2474BX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50334268 (CHEMBL1642655 | CHEMBL2205637 | N-(6-(1H-imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of TAK1 | Bioorg Med Chem Lett 22: 7326-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.084 BindingDB Entry DOI: 10.7270/Q2M32WXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50429867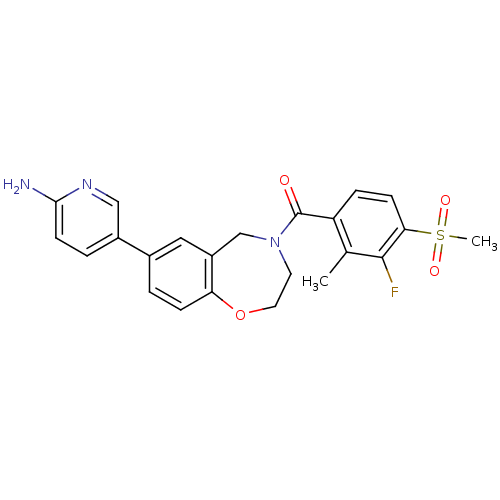 (CHEMBL2333365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis Curated by ChEMBL | Assay Description Inhibition of TAK1 (unknown origin) | J Med Chem 56: 2218-34 (2013) Article DOI: 10.1021/jm3007933 BindingDB Entry DOI: 10.7270/Q2ZC847X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50432373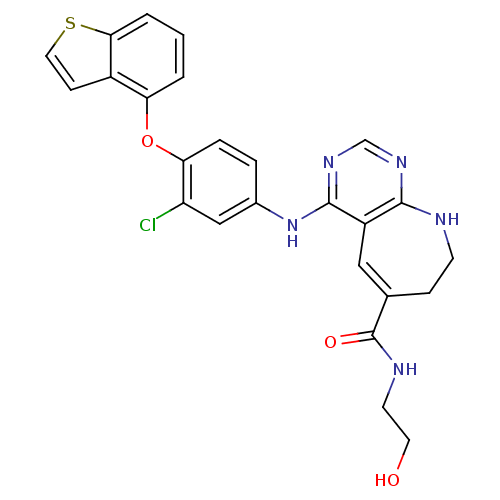 (CHEMBL2348417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged TAK1 (unknown origin) expressed in baculovirus expression system incubated for 5 mins prior to [gamma-33P]ATP ad... | Bioorg Med Chem 21: 2250-61 (2013) Article DOI: 10.1016/j.bmc.2013.02.014 BindingDB Entry DOI: 10.7270/Q2TT4S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50357884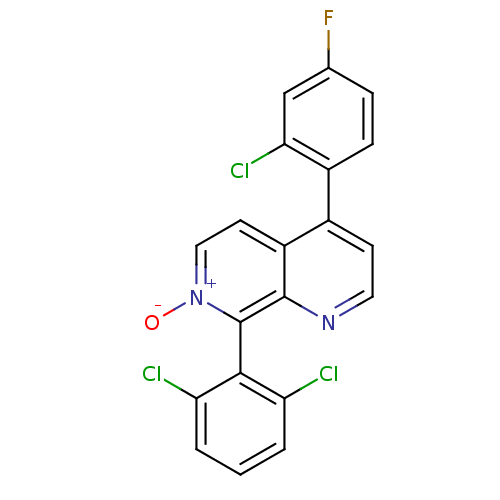 (CHEMBL1916359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibition of human TAK1 | J Med Chem 54: 7899-910 (2011) Article DOI: 10.1021/jm200975u BindingDB Entry DOI: 10.7270/Q2MW2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50358430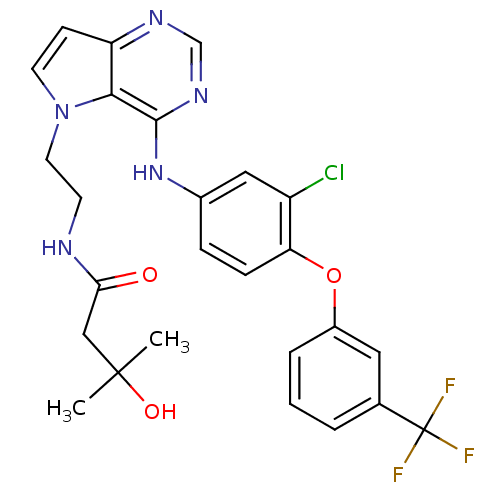 (CHEMBL1614725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminus flag-tagged TAK1 expressed in baculovirus infected insect cells using [gamma33P]ATP as substrate after 60 mins by scintillat... | J Med Chem 54: 8030-50 (2011) Article DOI: 10.1021/jm2008634 BindingDB Entry DOI: 10.7270/Q23R0T93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM357013 (5-Phenyl-N-(pyridin-2-ylmethyl)-2-(pyrimidin-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The analyses were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and subst... | US Patent US10214511 (2019) BindingDB Entry DOI: 10.7270/Q29Z975M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 2 group C member 2 (Homo sapiens (Human)) | BDBM50239229 (CHEMBL4097255 | US10676460, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The analyses were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and subst... | US Patent US10676460 (2020) BindingDB Entry DOI: 10.7270/Q2QZ2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||