

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50167290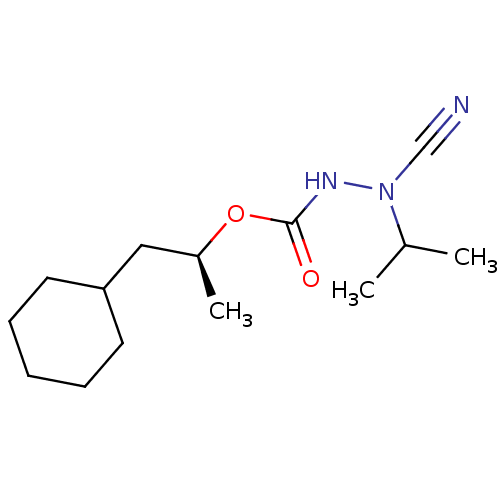 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin H using L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50167289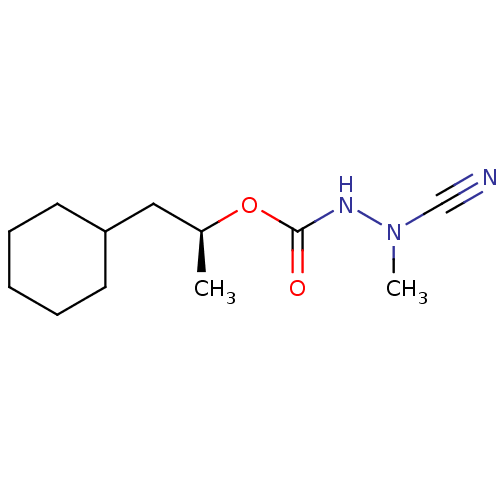 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin H using L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50247192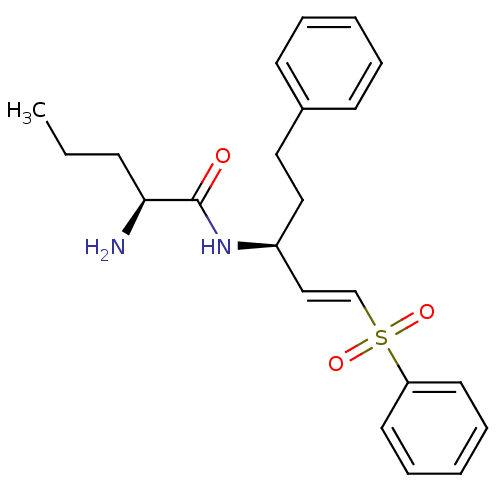 ((S)-2-amino-N-((S)-5-phenyl-1-(phenylsulfonyl)pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50464764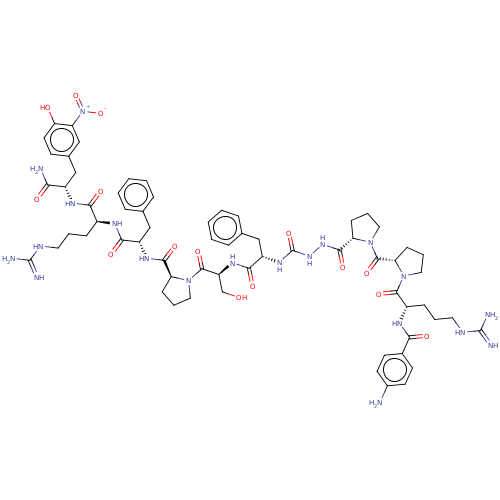 (CHEMBL4287663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 4301 Curated by ChEMBL | Assay Description Reversible competitive inhibition of human cathepsin H using fluorogenic AMC-derived peptide substrate assessed as reduction in residual activity pre... | Eur J Med Chem 144: 201-210 (2018) Article DOI: 10.1016/j.ejmech.2017.12.012 BindingDB Entry DOI: 10.7270/Q2MP55ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50243232 (CHEMBL486232 | GNF-PF-5434 | N-((S)-4-methyl-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50186088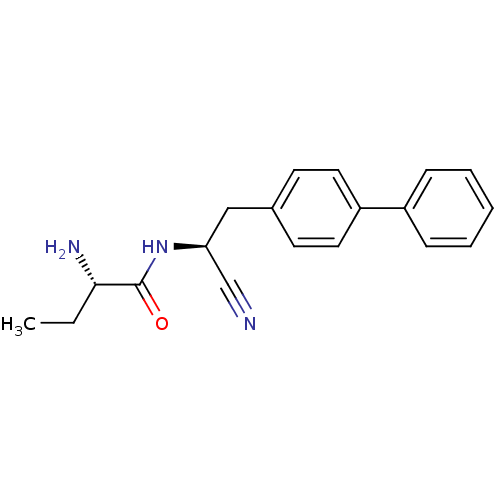 ((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50464766 (CHEMBL4292287) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 4301 Curated by ChEMBL | Assay Description Reversible competitive inhibition of human cathepsin H using fluorogenic AMC-derived peptide substrate assessed as reduction in residual activity pre... | Eur J Med Chem 144: 201-210 (2018) Article DOI: 10.1016/j.ejmech.2017.12.012 BindingDB Entry DOI: 10.7270/Q2MP55ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kurukshetra University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin H | Bioorg Med Chem 22: 4233-45 (2014) Article DOI: 10.1016/j.bmc.2014.05.037 BindingDB Entry DOI: 10.7270/Q25Q4XQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kurukshetra University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin H | Eur J Med Chem 77: 231-42 (2014) Article DOI: 10.1016/j.ejmech.2014.03.007 BindingDB Entry DOI: 10.7270/Q2319XDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50167297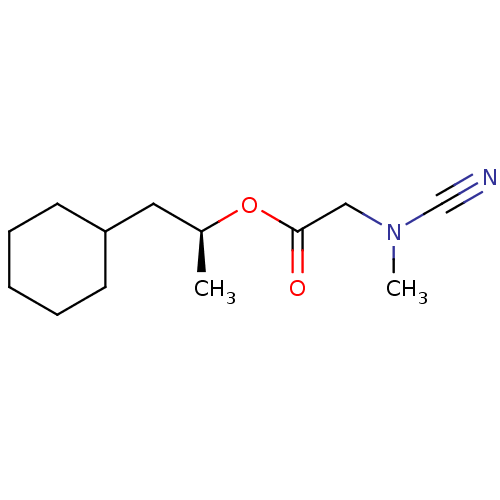 ((Cyano-methyl-amino)-acetic acid (S)-2-cyclohexyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin H using L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50460480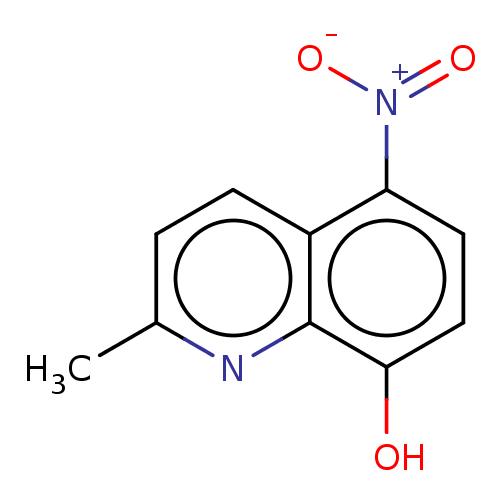 (CHEMBL4229176) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Non-competitive inhibition of human liver cathepsin H assessed as inhibitory constant for enzyme-inhibitor complex using R-AMC as substrate in presen... | Bioorg Med Chem Lett 28: 1239-1247 (2018) Article DOI: 10.1016/j.bmcl.2018.02.042 BindingDB Entry DOI: 10.7270/Q218394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50460480 (CHEMBL4229176) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Non-competitive inhibition of human liver cathepsin H assessed as inhibitory constant for enzyme-substrate-inhibitor complex using R-AMC as substrate... | Bioorg Med Chem Lett 28: 1239-1247 (2018) Article DOI: 10.1016/j.bmcl.2018.02.042 BindingDB Entry DOI: 10.7270/Q218394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50074051 (3-(1-Benzyloxycarbonyl-3-methyl-butylcarbamoyl)-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University of Freiburg Curated by ChEMBL | Assay Description Inhibition constant for the non time dependent inhibition of cathepsin H | J Med Chem 42: 560-72 (1999) Article DOI: 10.1021/jm981061z BindingDB Entry DOI: 10.7270/Q2M907TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50460493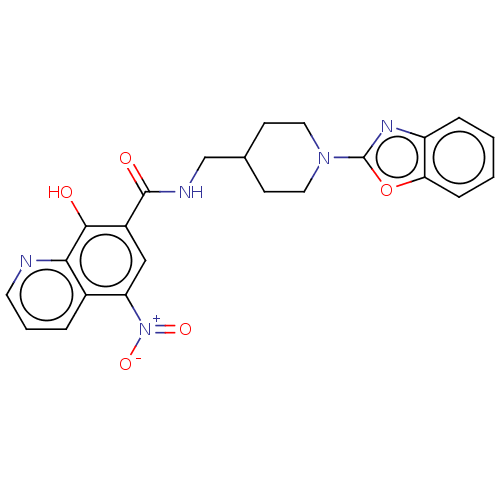 (CHEMBL4228921) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of human liver cathepsin H assessed as inhibitory constant for enzyme-inhibitor complex using R-AMC as substrate in presence o... | Bioorg Med Chem Lett 28: 1239-1247 (2018) Article DOI: 10.1016/j.bmcl.2018.02.042 BindingDB Entry DOI: 10.7270/Q218394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50425030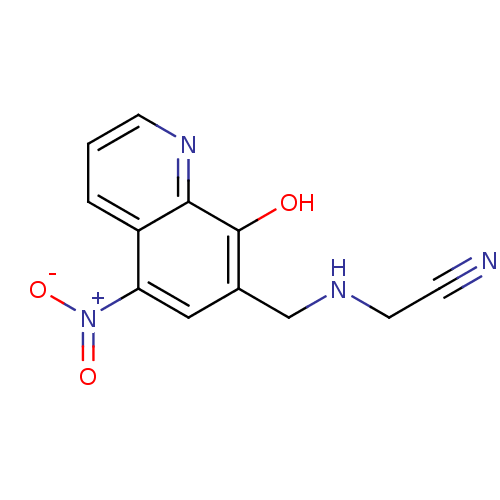 (CHEMBL2312216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Noncompetitive inhibition of human liver cathepsin H endopeptidase activity using R-AMC substrate assessed as inhibition constant for enzyme-inhibito... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50425030 (CHEMBL2312216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Noncompetitive inhibition of human liver cathepsin H endopeptidase activity using R-AMC substrate assessed as inhibition constant for enzyme-substrat... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50074050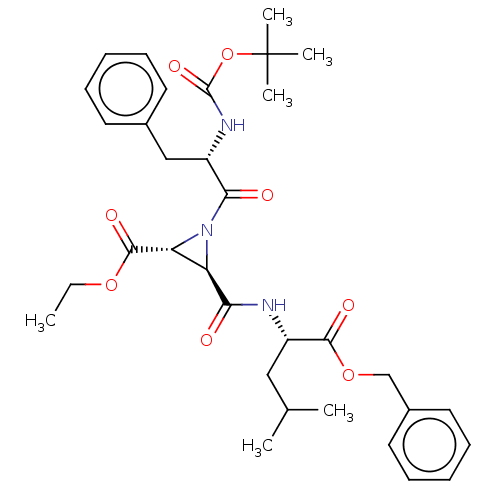 (3-(1-Benzyloxycarbonyl-3-methyl-butylcarbamoyl)-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University of Freiburg Curated by ChEMBL | Assay Description Inhibition constant for the non time dependent inhibition of cathepsin H | J Med Chem 42: 560-72 (1999) Article DOI: 10.1021/jm981061z BindingDB Entry DOI: 10.7270/Q2M907TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50074052 (1-(2-Benzyloxycarbonylamino-propionyl)-aziridine-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University of Freiburg Curated by ChEMBL | Assay Description Inhibition constant for the non time dependent inhibition of Cathepsin H | J Med Chem 42: 560-72 (1999) Article DOI: 10.1021/jm981061z BindingDB Entry DOI: 10.7270/Q2M907TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50074048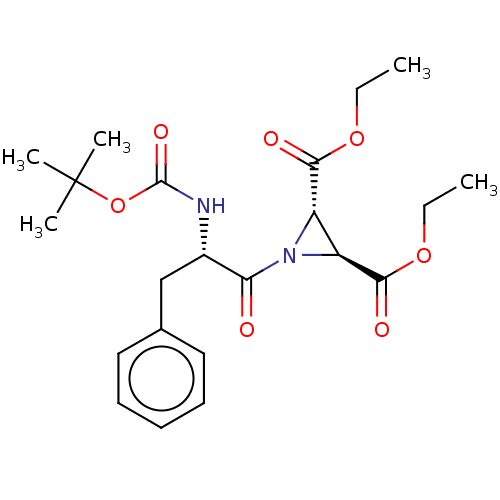 (1-((S)-2-tert-Butoxycarbonylamino-3-phenyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University of Freiburg Curated by ChEMBL | Assay Description Compound was evaluated for inhibition constant for the non-time dependent inhibition of Cathepsin H. | J Med Chem 42: 560-72 (1999) Article DOI: 10.1021/jm981061z BindingDB Entry DOI: 10.7270/Q2M907TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50425024 (CHEMBL2312204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Noncompetitive inhibition of human liver cathepsin H endopeptidase activity using R-AMC substrate assessed as inhibition constant for enzyme-inhibito... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50425024 (CHEMBL2312204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Noncompetitive inhibition of human liver cathepsin H endopeptidase activity using R-AMC substrate assessed as inhibition constant for enzyme-substrat... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50415097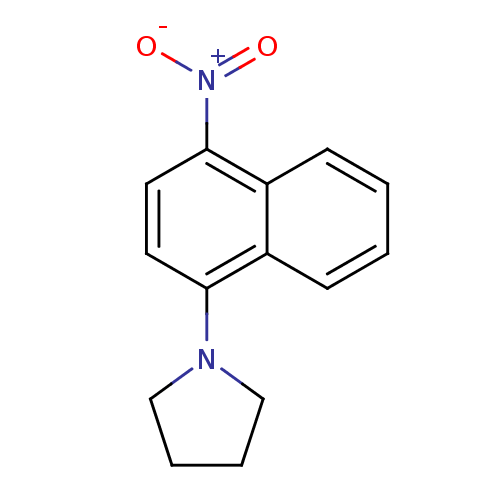 (CHEMBL570897) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Uncompetitive inhibition of human liver cathepsin H endopeptidase activity using R-AMC substrate assessed as inhibition constant for enzyme-substrate... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50074049 (3-((S)-1-Benzyloxycarbonyl-3-methyl-butylcarbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University of Freiburg Curated by ChEMBL | Assay Description Inhibition constant for the non time dependent inhibition of cathepsin H | J Med Chem 42: 560-72 (1999) Article DOI: 10.1021/jm981061z BindingDB Entry DOI: 10.7270/Q2M907TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50460502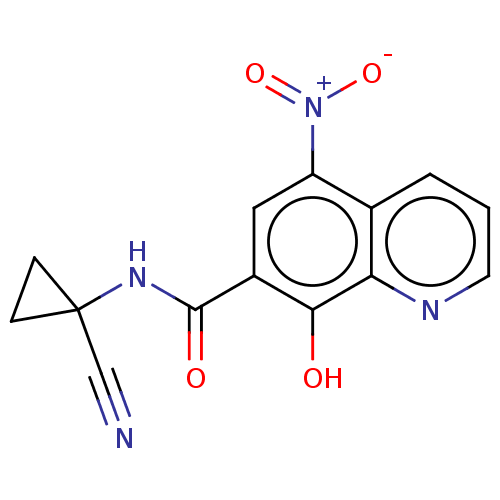 (CHEMBL4228576) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Non-competitive inhibition of human liver cathepsin H assessed as inhibitory constant for enzyme-inhibitor complex using R-AMC as substrate in presen... | Bioorg Med Chem Lett 28: 1239-1247 (2018) Article DOI: 10.1016/j.bmcl.2018.02.042 BindingDB Entry DOI: 10.7270/Q218394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50460502 (CHEMBL4228576) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Non-competitive inhibition of human liver cathepsin H assessed as inhibitory constant for enzyme-substrate-inhibitor complex using R-AMC as substrate... | Bioorg Med Chem Lett 28: 1239-1247 (2018) Article DOI: 10.1016/j.bmcl.2018.02.042 BindingDB Entry DOI: 10.7270/Q218394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50425017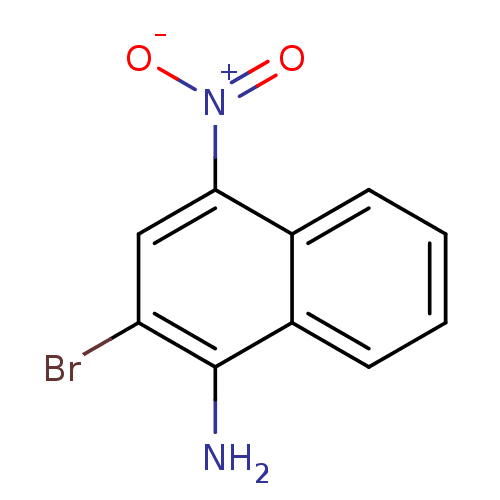 (CHEMBL2312203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 3.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Uncompetitive inhibition of human liver cathepsin H endopeptidase activity using R-AMC substrate assessed as inhibition constant for enzyme-substrate... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50460481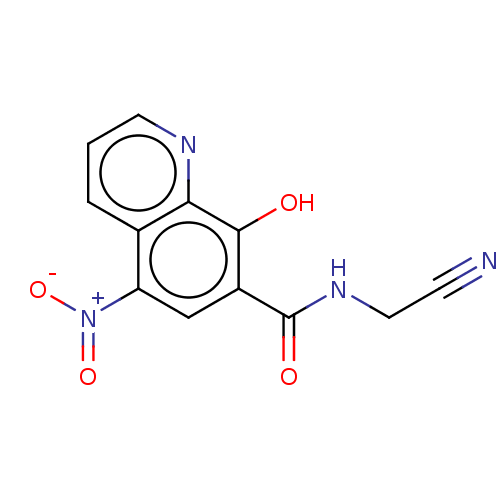 (CHEMBL4228126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Uncompetitive inhibition of human liver cathepsin H assessed as inhibitory constant for enzyme-substrate-inhibitor complex using R-AMC as substrate i... | Bioorg Med Chem Lett 28: 1239-1247 (2018) Article DOI: 10.1016/j.bmcl.2018.02.042 BindingDB Entry DOI: 10.7270/Q218394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||