

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374633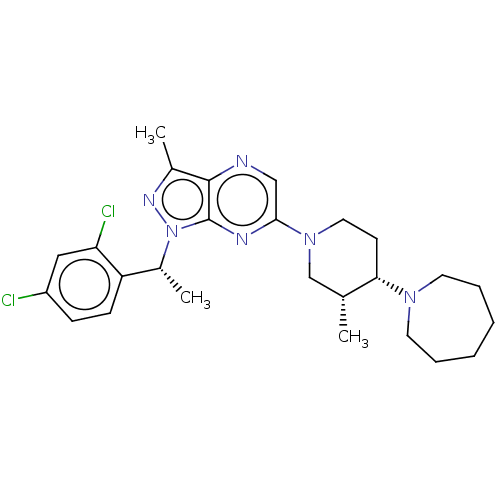 (6-((3R,4S)-4-(Azepan-1-yl)-3-methylpiperidin-1-yl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374652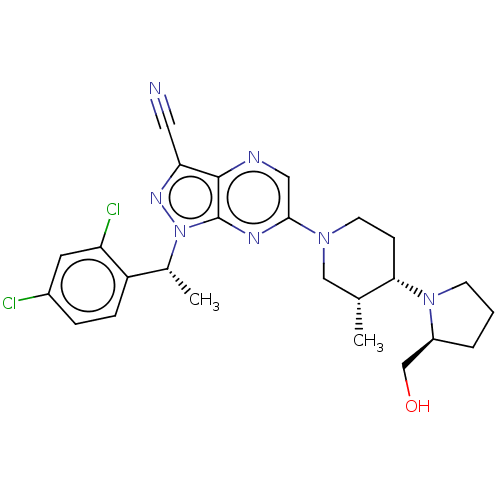 (1-((R)-1-(2,4-Dichlorophenyl)ethyl)-6-((3R,4S)-4-(...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374650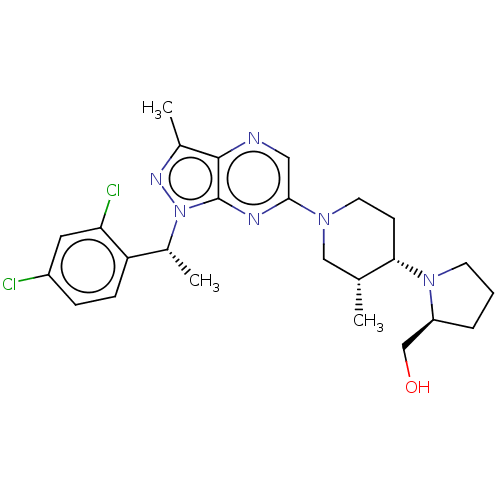 (((S)-1-((3R,4S)-1-(1-((R)-1-(2,4-Dichlorophenyl)et...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516634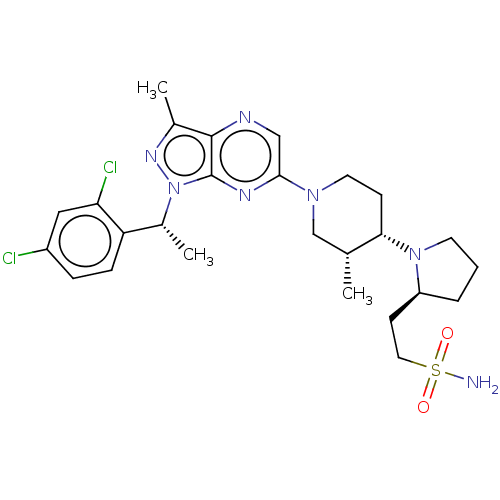 (CHEMBL4473604) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516626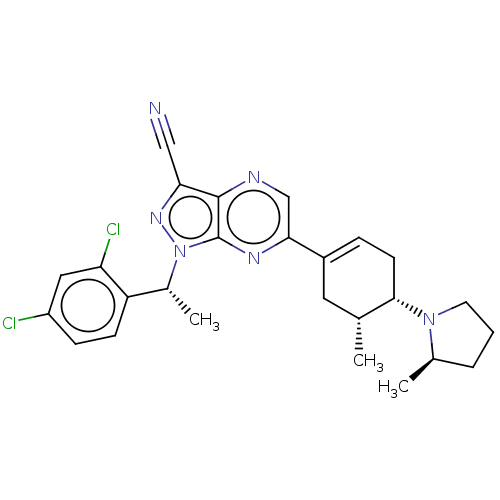 (CHEMBL4475665) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516632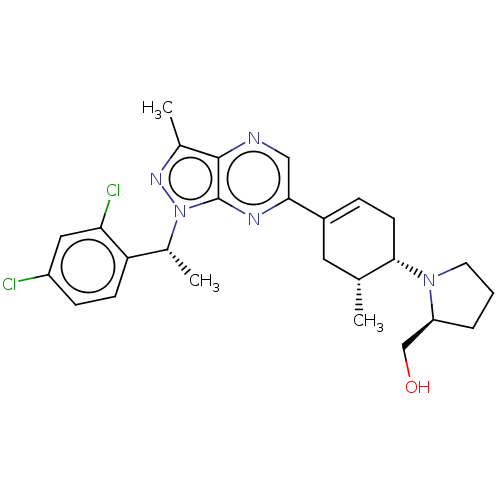 (CHEMBL4443688) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516623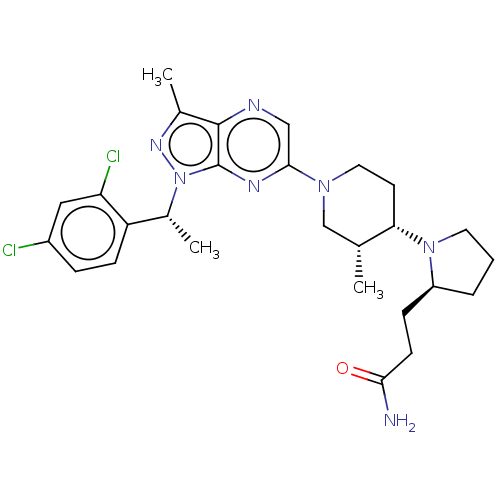 (CHEMBL4575089) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516625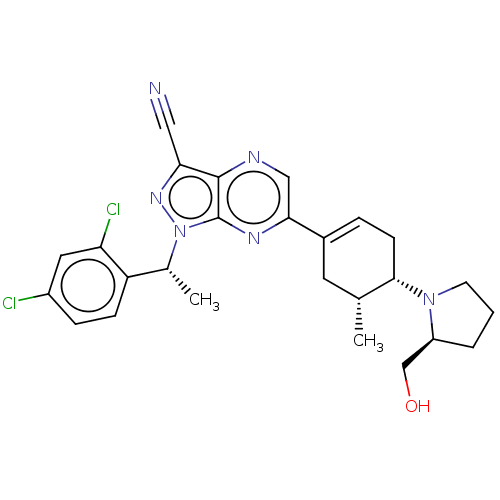 (CHEMBL4459231) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516635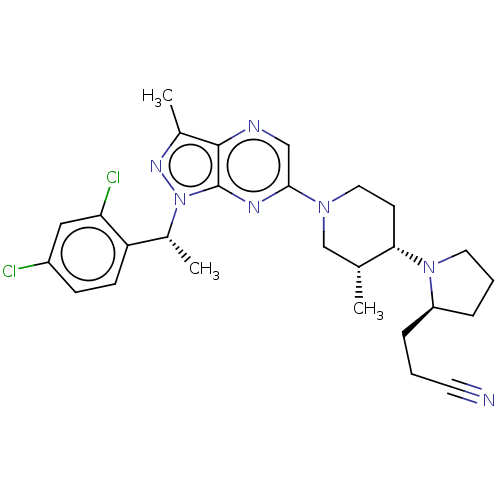 (CHEMBL4518040) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516633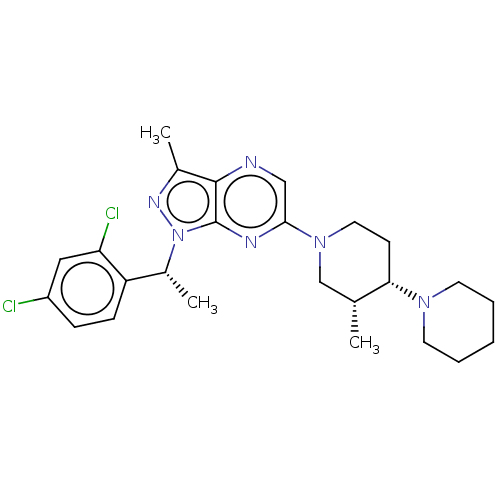 (CHEMBL4465359) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516626 (CHEMBL4475665) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374639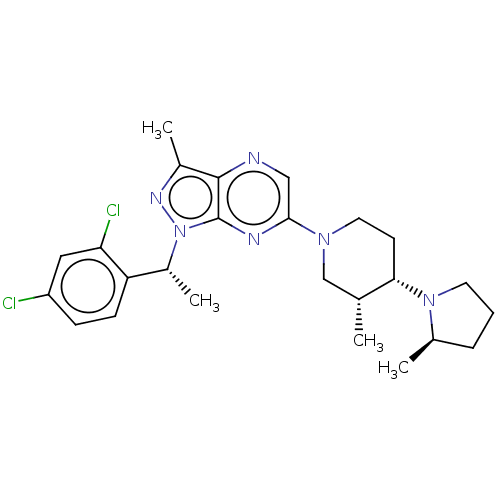 (1-((R)-1-(2,4-Dichlorophenyl)ethyl)-3-methyl-6-((3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516621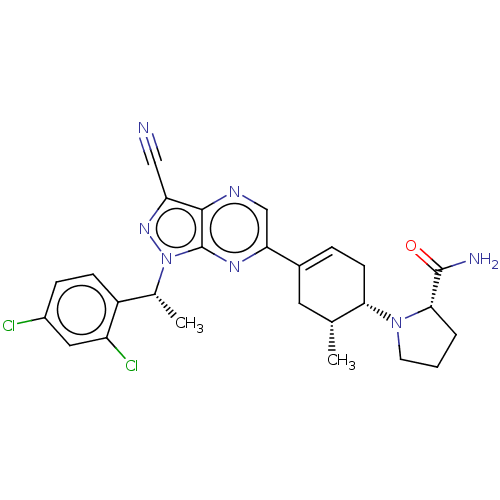 (CHEMBL4483537) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516627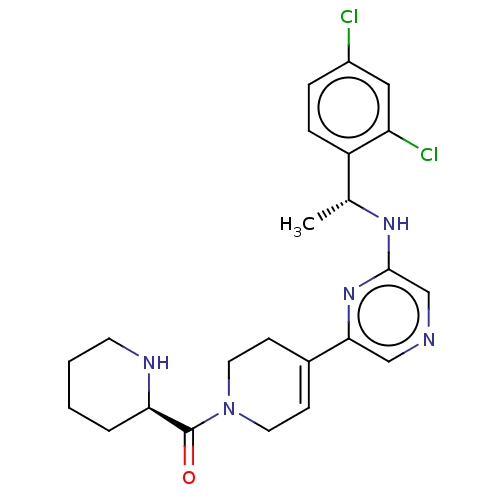 (CHEMBL4522437) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374652 (1-((R)-1-(2,4-Dichlorophenyl)ethyl)-6-((3R,4S)-4-(...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374632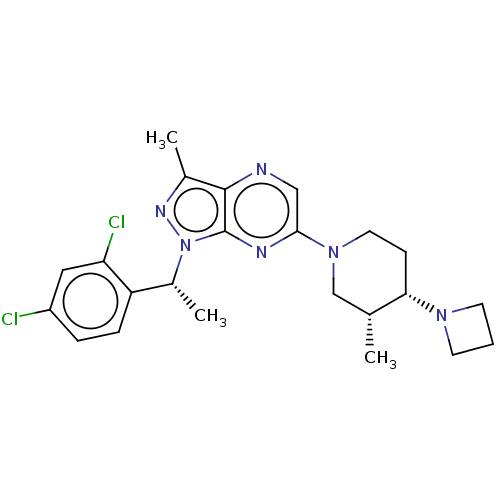 (6-((3R,4S)-4-(Azetidin-1-yl)-3-methylpiperidin-1-y...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516629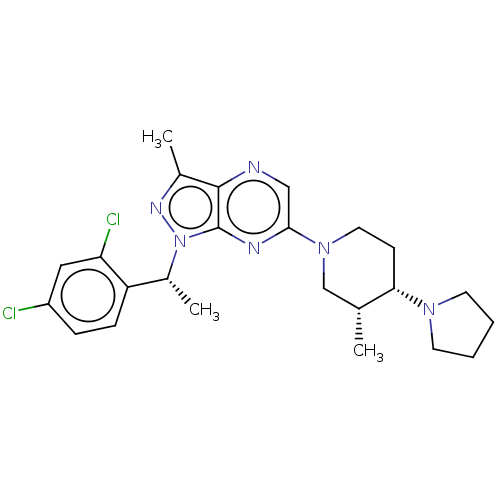 (CHEMBL4566304) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516625 (CHEMBL4459231) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516631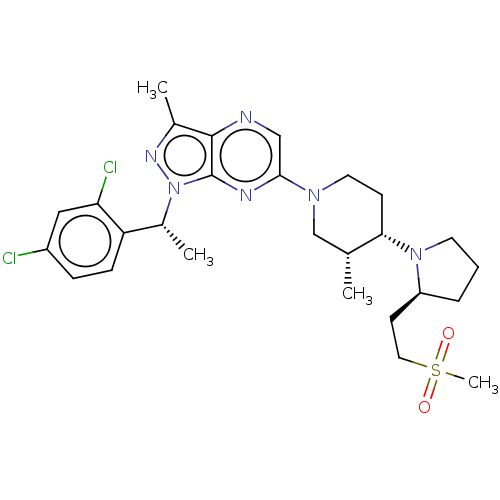 (CHEMBL4448901) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516628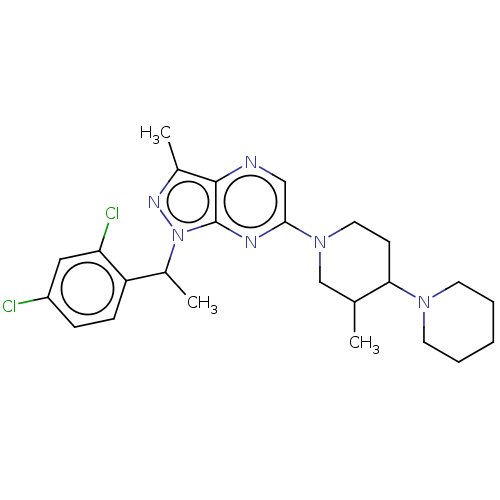 (CHEMBL4522547) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374650 (((S)-1-((3R,4S)-1-(1-((R)-1-(2,4-Dichlorophenyl)et...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50516625 (CHEMBL4459231) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in anti-CD3/CD28 stimulated mouse Treg cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% huma... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516630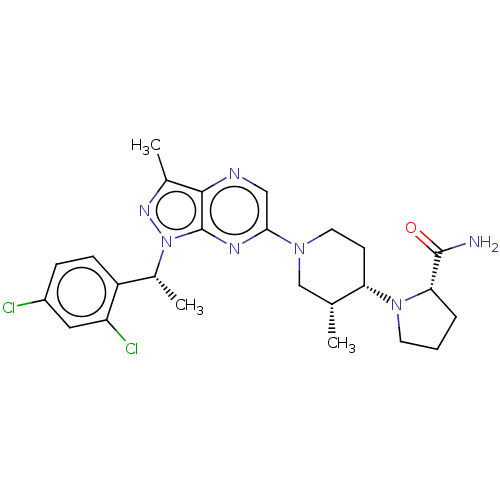 (CHEMBL4583503) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374641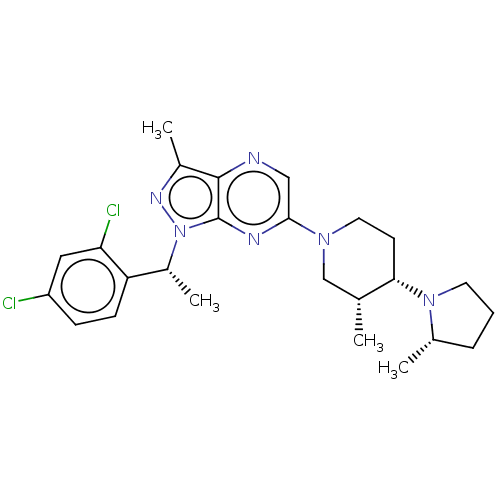 (1-((R)-1-(2,4-Dichlorophenyl)ethyl)-3-methyl-6-((3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516632 (CHEMBL4443688) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374633 (6-((3R,4S)-4-(Azepan-1-yl)-3-methylpiperidin-1-yl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374632 (6-((3R,4S)-4-(Azetidin-1-yl)-3-methylpiperidin-1-y...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374623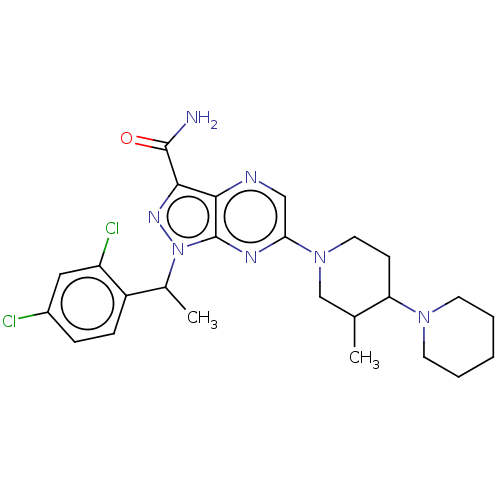 (1-(1-(2,4-Dichlorophenyl)ethyl)-6-(3′-methyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516624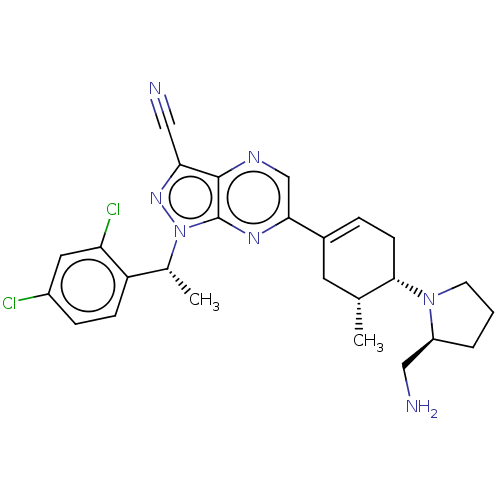 (CHEMBL4455432) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374627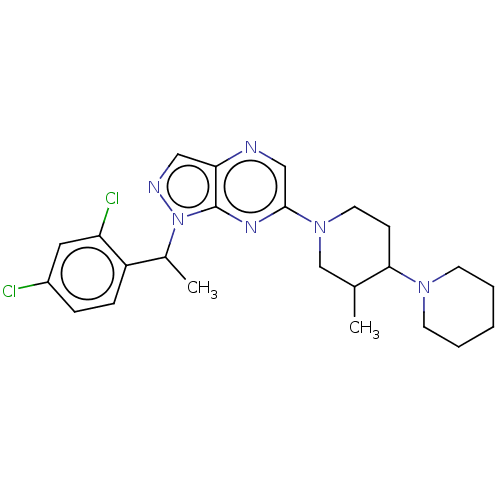 (1-(1-(2,4-Dichlorophenyl)ethyl)-6-(3′-methyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516622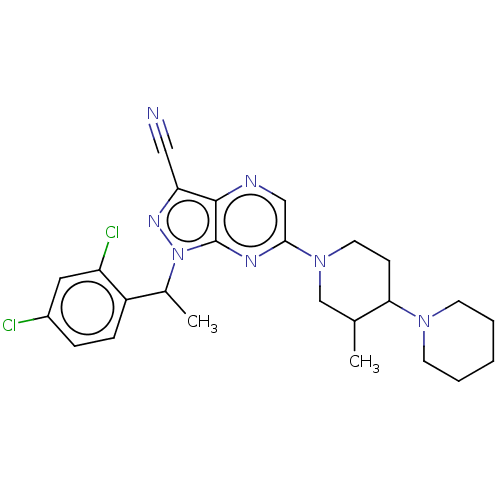 (CHEMBL4566241) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516634 (CHEMBL4473604) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516638 (CHEMBL4437594) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50516626 (CHEMBL4475665) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in anti-CD3/CD28 stimulated mouse Treg cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% huma... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516623 (CHEMBL4575089) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516633 (CHEMBL4465359) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374639 (1-((R)-1-(2,4-Dichlorophenyl)ethyl)-3-methyl-6-((3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516636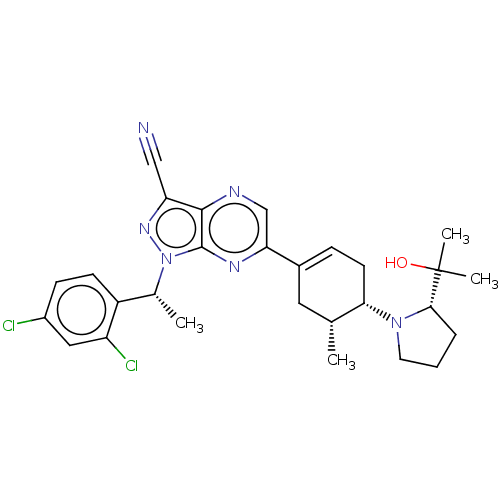 (CHEMBL4555534) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516624 (CHEMBL4455432) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 638 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374653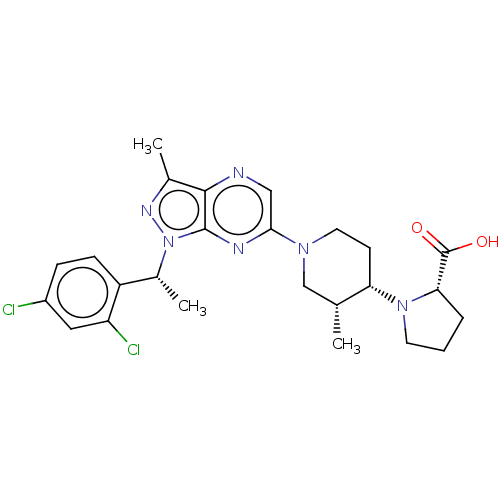 (((3R,4S)-1-(1-((R)-1-(2,4-Dichlorophenyl)ethyl)-3-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516640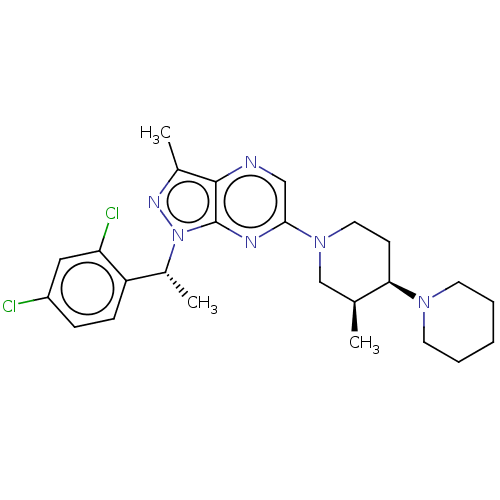 (CHEMBL4537681) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 803 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374629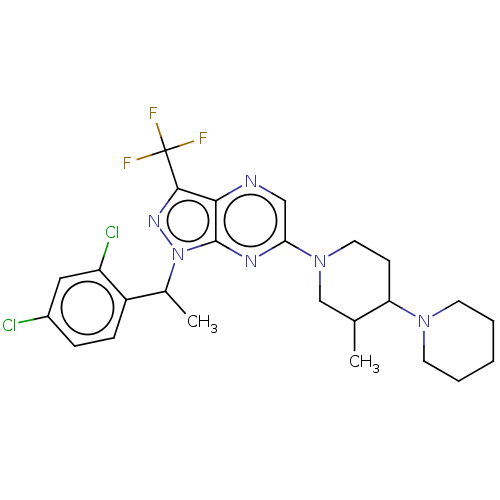 (1-(1-(2,4-Dichlorophenyl)ethyl)-6-(3′-methyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 837 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516635 (CHEMBL4518040) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 865 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516636 (CHEMBL4555534) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516621 (CHEMBL4483537) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis in presence of 100% human serum pre-incubated... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516637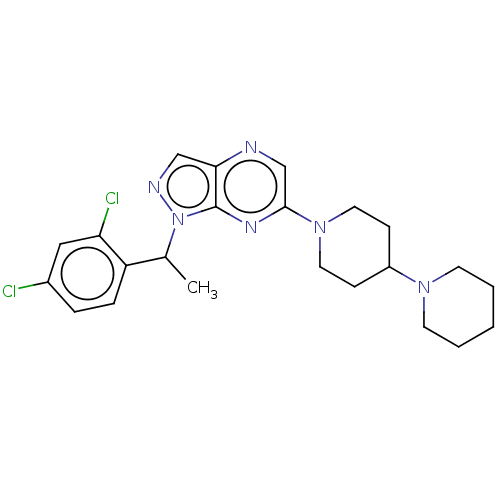 (CHEMBL4446924) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516639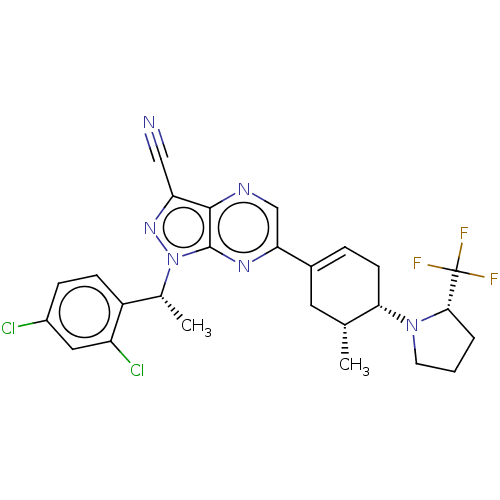 (CHEMBL4459504) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM374652 (1-((R)-1-(2,4-Dichlorophenyl)ethyl)-6-((3R,4S)-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50516625 (CHEMBL4459231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50516625 (CHEMBL4459231) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |