

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50518036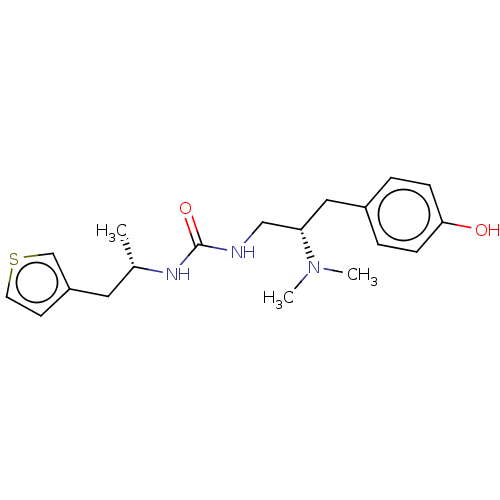 (CHEMBL4467777 | US11484525, Compound BD-122LS-PZM2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from mu opioid receptor (unknown origin) expressed in sf9 insect cell membranes after 1 hr by liquid scintillation... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50518026 (CHEMBL4449152) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from mu opioid receptor (unknown origin) expressed in sf9 insect cell membranes after 1 hr by liquid scintillation... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50518058 (CHEMBL4463417) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from mu opioid receptor (unknown origin) expressed in sf9 insect cell membranes after 1 hr by liquid scintillation... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50061101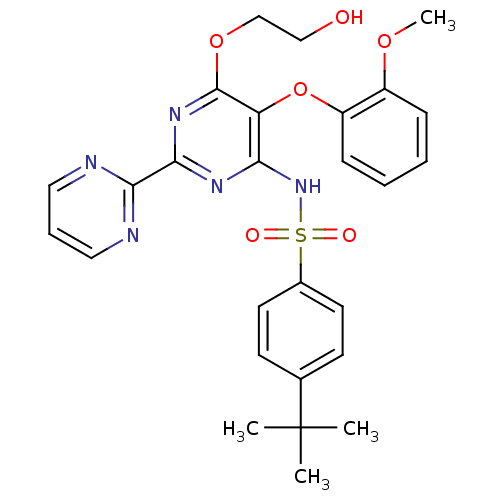 (4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Antagonist activity at ETB receptor (unknown origin) assessed as effect on G protein-mediated smooth muscle contraction | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50061101 (4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Antagonist activity at ETA receptor (unknown origin) assessed as increase in G protein-mediated vasoconstriction | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50518021 (CHEMBL610279) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at full-length Renilla luciferase 8 fused with c-terminal human kappa opioid receptor expressed in HEK293T cells assessed as inhibit... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50518022 (CHEMBL4458783) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membranes assessed as increase in [35S]-GTPgammaS binding after 1 hrs by TopCou... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50518023 (CHEMBL4513533 | US10751335, Example 14) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in human U2OS cells co-transfected with beta-arrestin-2 assessed as increase in beta-arrestin-... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50389129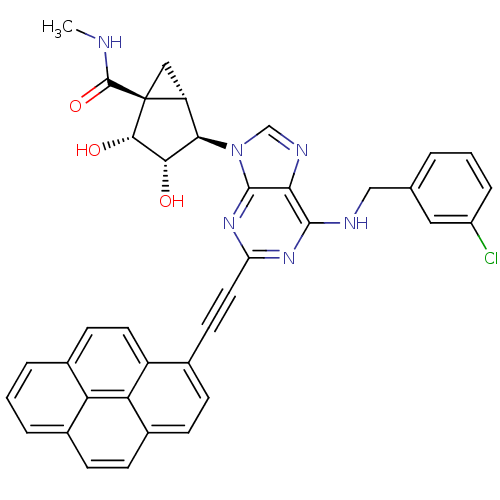 (CHEMBL2064661) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at adenosine A3 receptor (unknown origin) expressed in serum starved CHO cells assessed as increase in cell survival after 24 hrs by... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM17657 ((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at mGlu5 receptor (unknown origin) expressed in CHO cells assessed as increase in Gq-mediated PI hydrolysis after 45 mins by yttrium... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM442801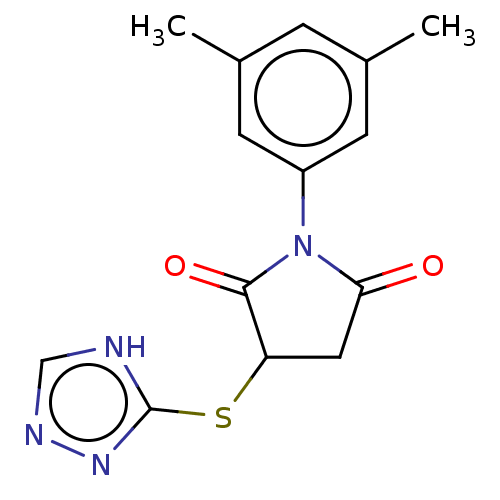 (WAY-308264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as reversal of Ro 20-1724 mediated decrease in cAMP accumulation aft... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Inverse agonist activity at dopamine D2 receptor (unknown origin) assessed as increase in beta-arrestin-2 recruitment | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50389151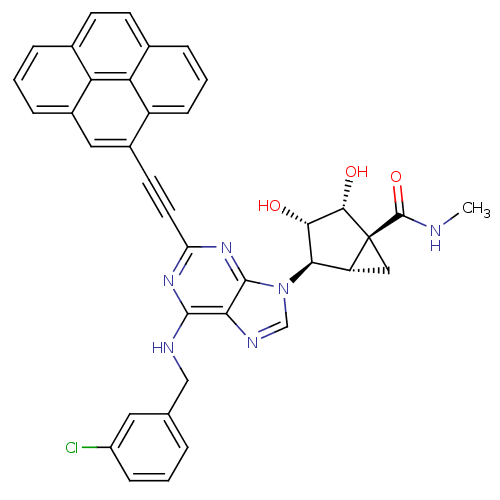 (CHEMBL2064634) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in FlpIn-CHO cells assessed as inhibition of forskolin-mediated cAMP accumulation preincuba... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM60917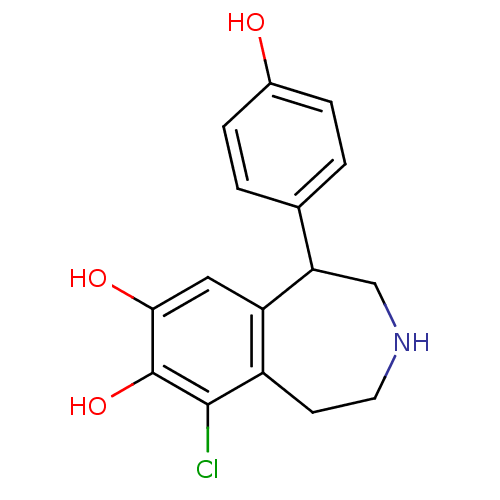 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as assessed as increase in beta-arrestin-2 recruitment after 60 mins... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50303526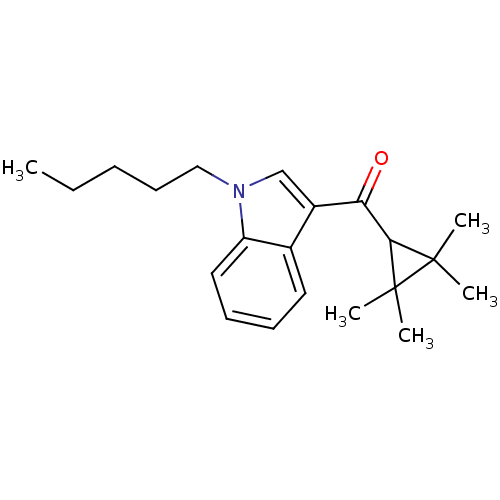 ((1-Pentyl-1H-indol-3-yl)(2,2,3,3-tetramethylcyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 95 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Inverse agonist activity at C-terminally prolink-tagged mouse CB2 receptor expressed in CHOK1 cells harboring beta-galactosidase enzyme fused beta-ar... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50518024 (CHEMBL4438370) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at GFP10 tagged human APJ receptor expressed in HEK293 cells harboring Rluc2-beta-arrestin1 assessed as increase in beta-arrestin1 r... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50387454 (CHEMBL2047474) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at histamine H4 receptor (unknown origin) expressed in human U2OS cells increase in beta-arrestin2 recruitment after 2 hrs by lumine... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50518025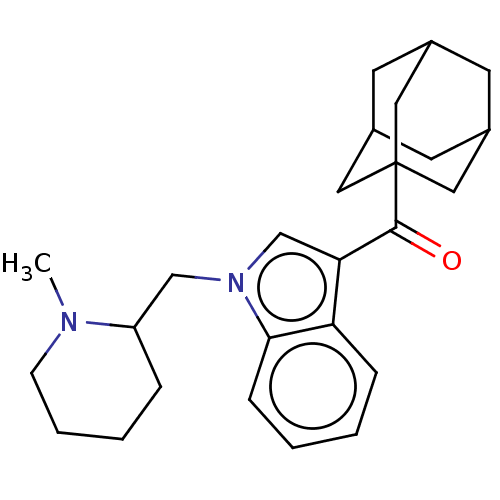 (CHEMBL4467282) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Inverse agonist activity at mouse CB2 receptor expressed in HEK293 cells assessed as inhibition of forskolin-mediated cAMP accumulation after 5 mins ... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at GPR109A (unknown origin) assessed as effect on beta-arrestin2 conformational changes | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50518026 (CHEMBL4449152) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at Gi/o coupled human mu opioid receptor expressed in HEK293T cells co-expressing luciferase based cAMP biosensor and GRK2 assessed ... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50518027 (CHEMBL4550815 | US11498896, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at dopamine D2long receptor (unknown origin) assessed as increased in cAMP accumulation | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50518028 (CHEMBL4472022) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as assessed as increase in beta-arrestin-2 recruitment after 60 mins... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004917 (3-Allyl-6-chloro-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as assessed as increase in beta-arrestin-2 recruitment after 60 mins... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50274531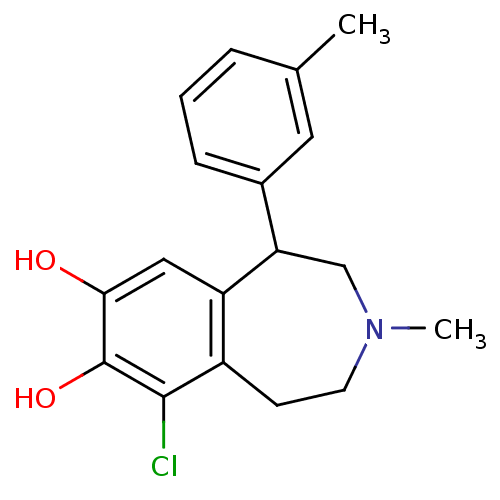 (6-Chloro-3-methyl-1-m-tolyl-2,3,4,5-tetrahydro-1H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as assessed as increase in beta-arrestin-2 recruitment after 60 mins... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004918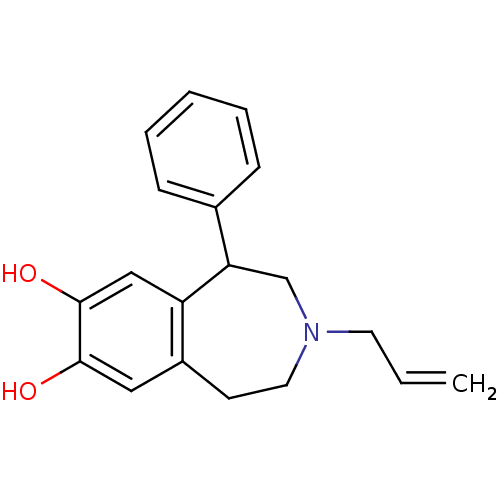 ((+/-)-APD3-Allyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as assessed as increase in beta-arrestin-2 recruitment after 60 mins... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM442801 (WAY-308264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as assessed as increase in beta-arrestin-2 recruitment after 60 mins... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86277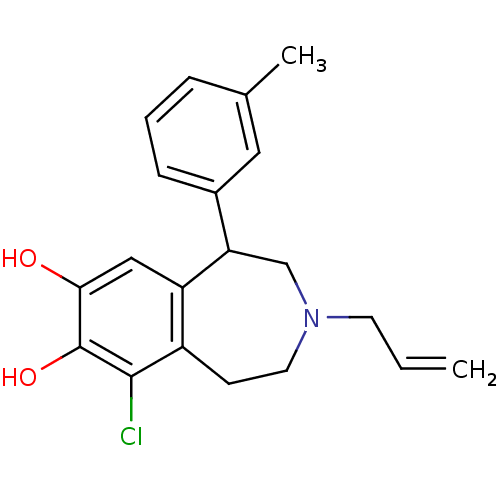 (MCL-210) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as reversal of Ro 20-1724 mediated decrease in cAMP accumulation aft... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50518028 (CHEMBL4472022) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as reversal of Ro 20-1724 mediated decrease in cAMP accumulation aft... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004917 (3-Allyl-6-chloro-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as reversal of Ro 20-1724 mediated decrease in cAMP accumulation aft... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50274531 (6-Chloro-3-methyl-1-m-tolyl-2,3,4,5-tetrahydro-1H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as reversal of Ro 20-1724 mediated decrease in cAMP accumulation aft... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM60917 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as reversal of Ro 20-1724 mediated decrease in cAMP accumulation aft... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50164848 (CHEMBL3798179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 154 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at dopamine D2 receptor (unknown origin) assessed as increase in beta-arrestin-2 recruitment | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50518029 (CHEMBL4442260) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at dopamine D2 receptor (unknown origin) assessed as increase in beta-arrestin-2 recruitment after 20 mins measured for 1 sec by BRE... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50518030 (CHEMBL4467404) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2long receptor (unknown origin) expressed in HEK293T cells co-expressing TEV fused-beta-Arrestin2 and GRK2 assess... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50518031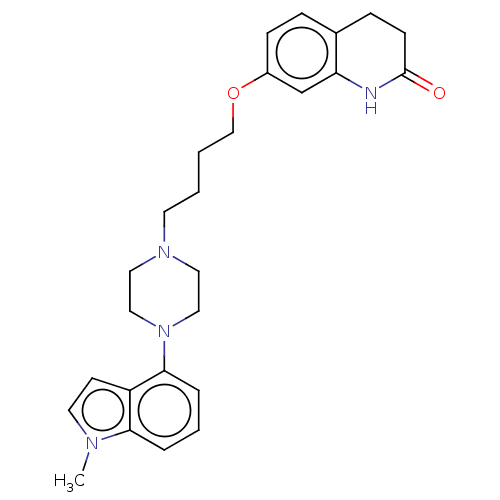 (CHEMBL4445841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2long receptor (unknown origin) expressed in HEK293T cells co-expressing TEV fused-beta-Arrestin2 and GRK2 assess... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50518030 (CHEMBL4467404) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at dopamine D2 receptor (unknown origin) expressed in HEK293T cells co-expressing luciferase based cAMP biosensor assessed as inhibi... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50518032 (CHEMBL4456531) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at YFP-fused human NOPR expressed in HEK293 cells assessed as inhibition of forskolin-mediated cAMP accumulation measured immediatel... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50255899 (1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3S)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human NOPR expressed in human BHK cells assessed as increase in beta-arrestin-2 recruitment | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50325534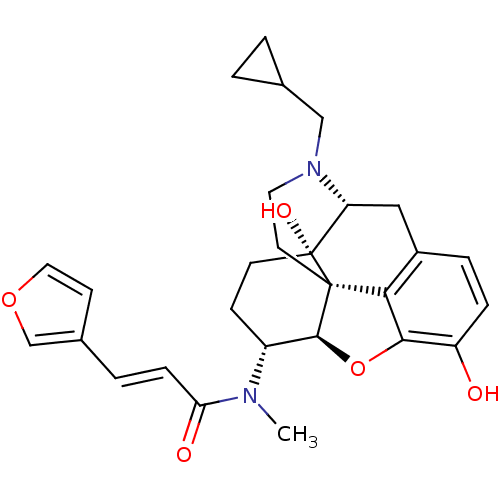 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged rat kappa opioid receptor expressed in HEK293 cells assessed as increase in beta-arrestin mediated p38 phosphorylation... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50325534 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged rat kappa opioid receptor expressed in HEK293 cells assessed as increase in ERK1/2 phosphorylation after 5 mins by Wes... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325534 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged human kappa opioid receptor expressed in HEK293 cells assessed as increase in ERK1/2 phosphorylation after 5 mins by ... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50491417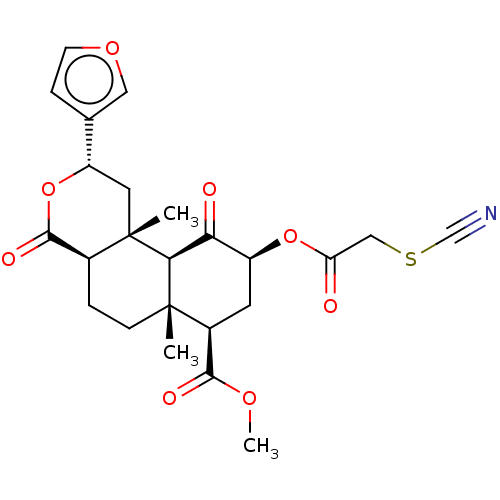 (22-THIOCYANATOSALVINORIN A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor (unknown origin) expressed in HEK cells co-expressing luciferase based cAMP biosensor assessed as increase ... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cell membranes after 1 hr by [35S]-GTPgammaS binding assay | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50518033 (CHEMBL4537962) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membranes assessed as increase in [35S]-GTPgammaS binding after 1 hrs by TopCou... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50518022 (CHEMBL4458783) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 329 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in human U2OS cells assessed as increase in ERK1/2 phosphorylation after 10 mins by Western... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130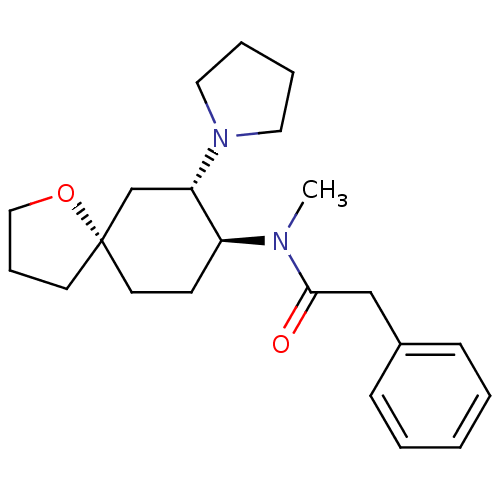 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 205 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in human U2OS cells co-transfected with GFP and beta-arrestin-2 assessed as increase in bet... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 131 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in human U2OS cells co-transfected with EFC and beta-arrestin-2 assessed as increase in bet... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50254705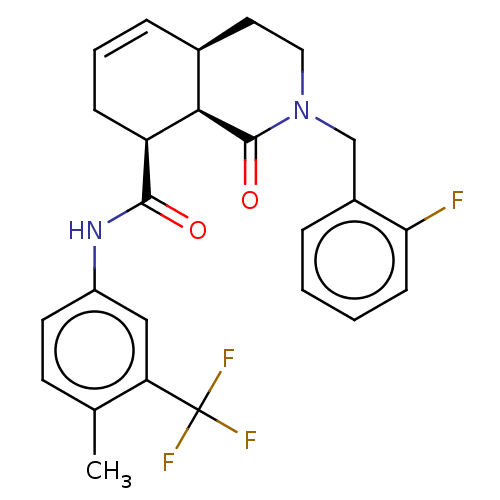 (CHEMBL4068633) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in human U2OS cells co-transfected with EFC and beta-arrestin-2 assessed as increase in bet... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817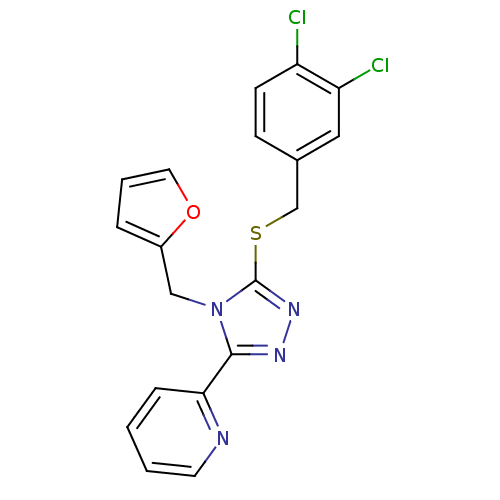 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in human U2OS cells co-transfected with EFC and beta-arrestin-2 assessed as increase in bet... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50518034 (CHEMBL4451128) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 374 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in human U2OS cells co-transfected with beta-arrestin-2 assessed as increase in beta-arrestin-... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 179 total ) | Next | Last >> |