

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120086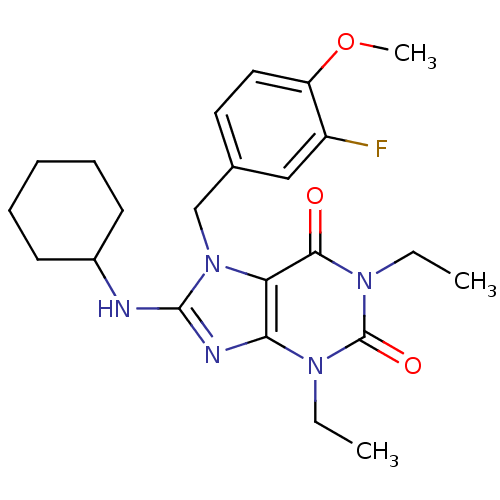 (8-Cyclohexylamino-1,3-diethyl-7-(3-fluoro-4-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120069 (8-Cyclohexylamino-1,3-diethyl-7-(3-fluoro-4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120079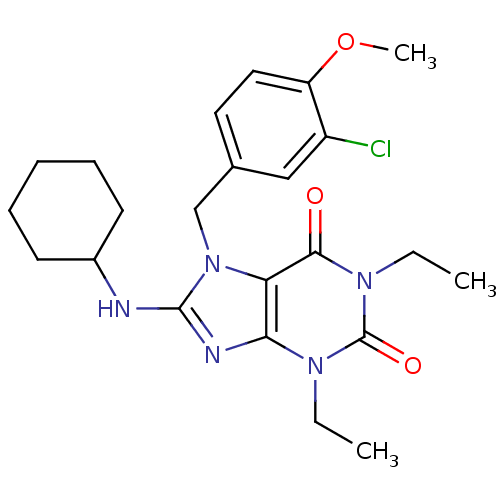 (7-(3-Chloro-4-methoxy-benzyl)-8-cyclohexylamino-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120074 (8-Cyclohexylamino-1,3-diethyl-7-(4-hydroxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120067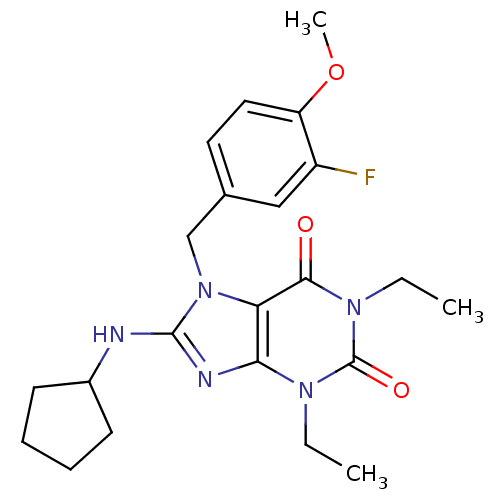 (8-Cyclopentylamino-1,3-diethyl-7-(3-fluoro-4-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120072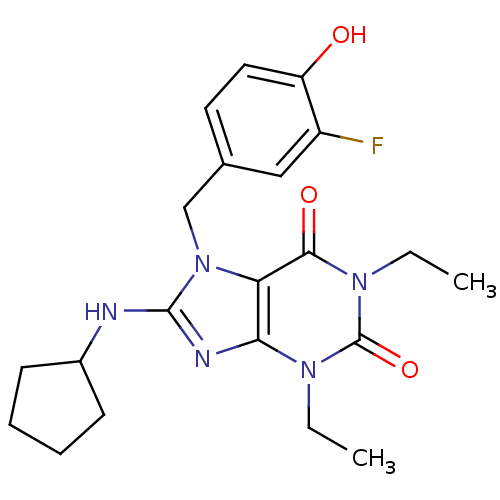 (8-Cyclopentylamino-1,3-diethyl-7-(3-fluoro-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120071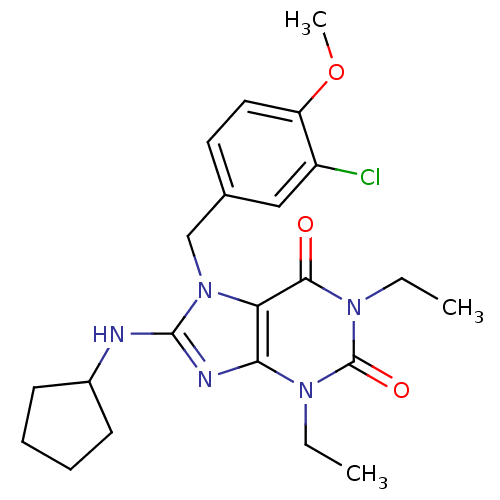 (7-(3-Chloro-4-methoxy-benzyl)-8-cyclopentylamino-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120080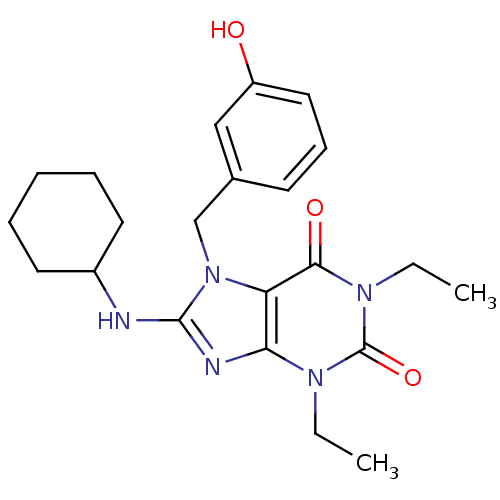 (8-Cyclohexylamino-1,3-diethyl-7-(3-hydroxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120070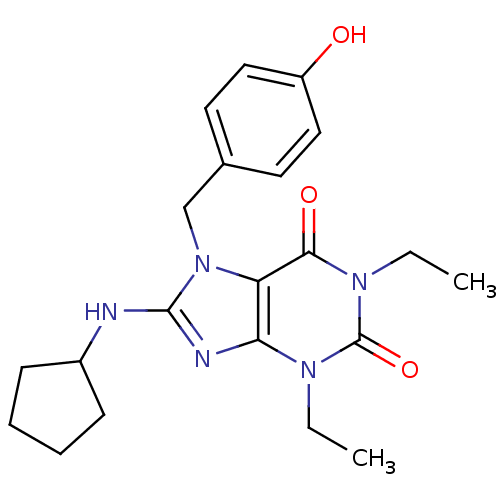 (8-Cyclopentylamino-1,3-diethyl-7-(4-methoxy-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120081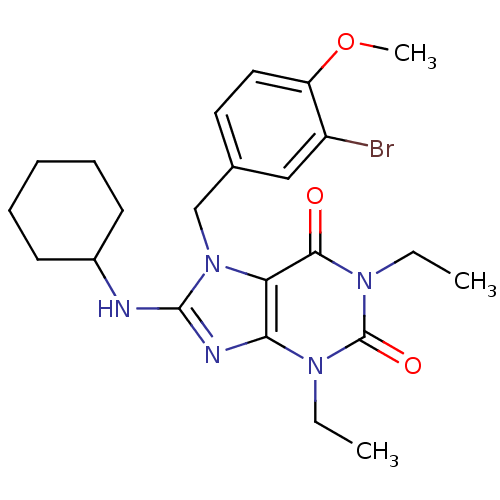 (7-(3-Bromo-4-methoxy-benzyl)-8-cyclohexylamino-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120078 (7-(3-Chloro-4-hydroxy-benzyl)-8-cyclopentylamino-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120085 (7-(3-Chloro-4-hydroxy-benzyl)-8-cyclohexylamino-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120068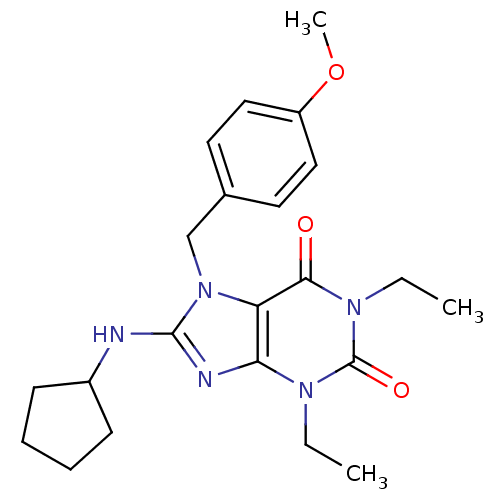 (8-Cyclopentylamino-1,3-diethyl-7-(4-methoxy-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120083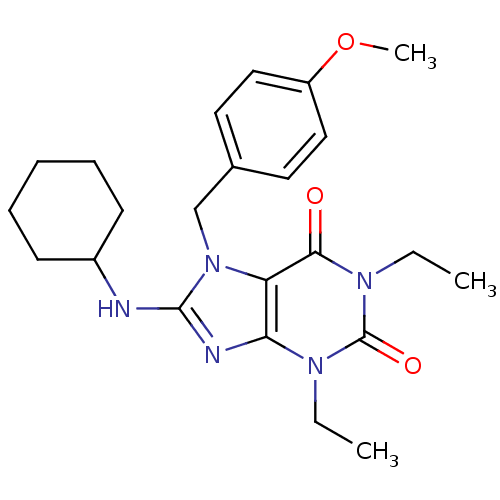 (8-Cyclohexylamino-1,3-diethyl-7-(4-methoxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120075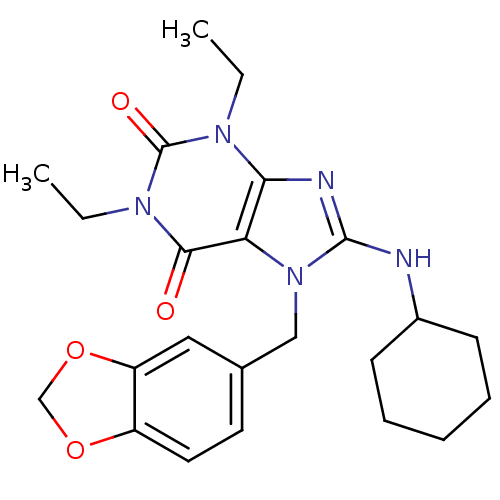 (7-Benzo[1,3]dioxol-5-ylmethyl-8-cyclohexylamino-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120076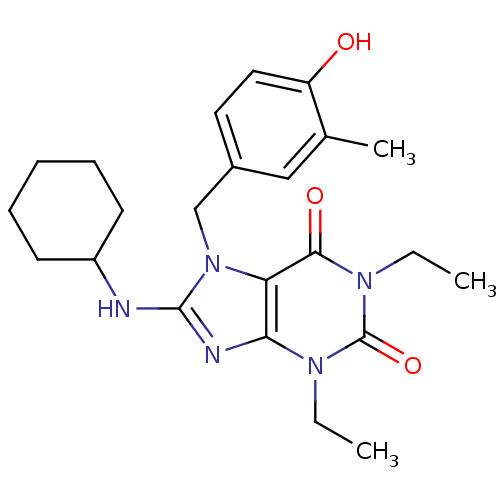 (8-Cyclohexylamino-1,3-diethyl-7-(4-hydroxy-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120073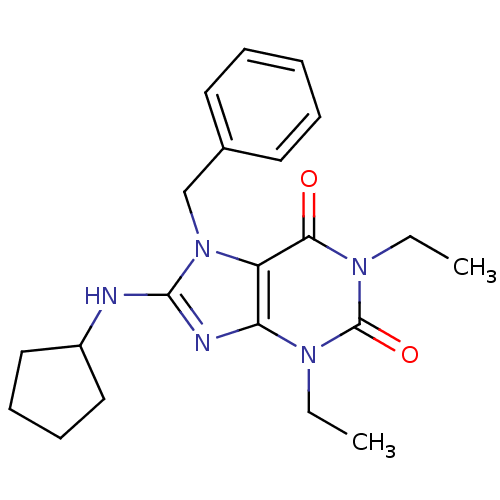 (7-Benzyl-8-cyclopentylamino-1,3-diethyl-3,7-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120082 (7-Benzyl-8-cyclohexylamino-1,3-diethyl-3,7-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120077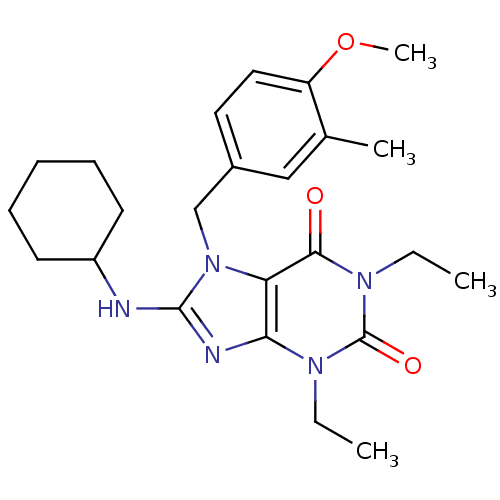 (8-Cyclohexylamino-1,3-diethyl-7-(4-methoxy-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120084 (8-Cyclohexylamino-1,3-diethyl-7-(3-methoxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||