 Found 48 hits Enz. Inhib. hit(s) with all data for entry = 50018236
Found 48 hits Enz. Inhib. hit(s) with all data for entry = 50018236 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407800
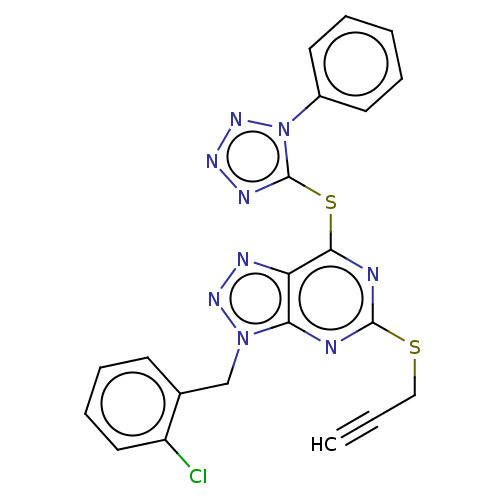
(CHEMBL5291138)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)[C@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C38H53N11O5S/c39-37(40)43-15-5-12-26-33(51)47-28(20-25-11-7-17-55-25)35(53)48-21-24-10-2-1-8-22(24)18-31(48)36(54)49-29-14-4-3-9-23(29)19-30(49)34(52)46-27(32(50)45-26)13-6-16-44-38(41)42/h1-2,7-8,10-11,17,23,26-31H,3-6,9,12-16,18-21H2,(H,45,50)(H,46,52)(H,47,51)(H4,39,40,43)(H4,41,42,44)/t23?,26-,27-,28-,29?,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346870
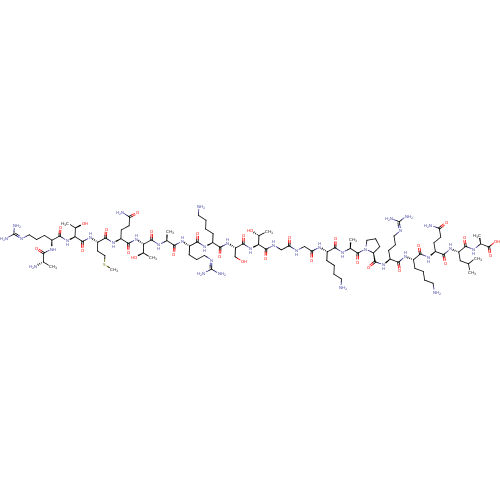
(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346870

(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586363
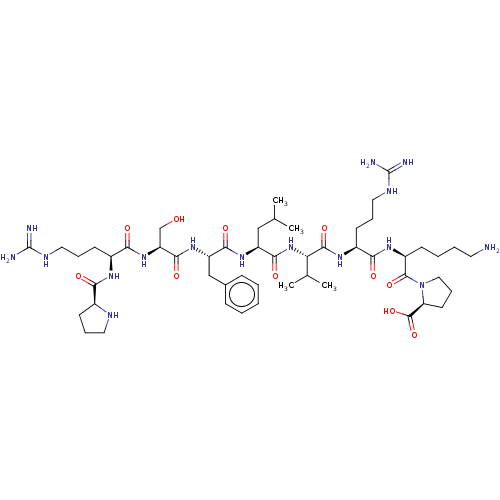
(CHEMBL5079374)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50407795

(CHEMBL5281080)Show SMILES C[C@@H]1NC(=O)[C@H](Cc2cccs2)NC(=O)CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C39H53N9O6S/c1-23-37(53)47-22-26-11-3-2-9-24(26)19-32(47)38(54)48-30-14-5-4-10-25(30)20-31(48)36(52)46-28(13-6-17-43-39(40)41)34(50)42-16-7-15-33(49)45-29(35(51)44-23)21-27-12-8-18-55-27/h2-3,8-9,11-12,18,23,25,28-32H,4-7,10,13-17,19-22H2,1H3,(H,42,50)(H,44,51)(H,45,49)(H,46,52)(H4,40,41,43)/t23-,25?,28-,29-,30?,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50407796
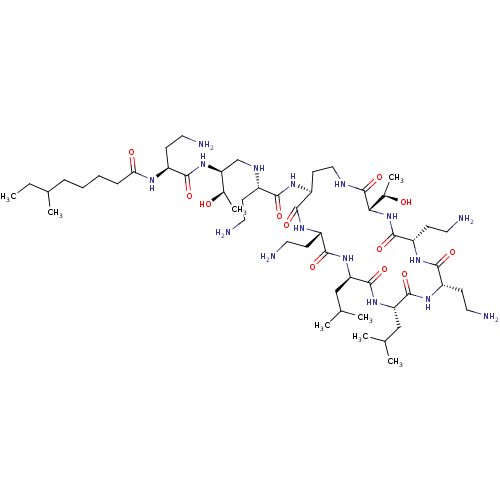
(CHEMBL1255711)Show SMILES [#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C36H48N8O6/c37-20-31(45)41-27(17-22-9-2-1-3-10-22)33(47)43-21-25-13-5-4-11-23(25)18-30(43)34(48)44-28-15-7-6-12-24(28)19-29(44)32(46)42-26(35(49)50)14-8-16-40-36(38)39/h1-5,9-11,13,24,26-30H,6-8,12,14-21,37H2,(H,41,45)(H,42,46)(H,49,50)(H4,38,39,40)/t24?,26-,27-,28?,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50407722

(CHEMBL5287743)Show InChI InChI=1S/C13H11N3OS2/c1-8-6-12(19-16-8)15-13(17)14-10-2-3-11-9(7-10)4-5-18-11/h2-7H,1H3,(H2,14,15,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407723
(CHEMBL5274037)Show InChI InChI=1S/C13H11N3O2S/c1-8-6-12(18-16-8)15-13(17)14-10-2-3-11-9(7-10)4-5-19-11/h2-7H,1H3,(H2,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50407721
(CHEMBL5266466)Show InChI InChI=1S/C14H14N4O2/c1-9-7-13(20-17-9)16-14(19)15-11-3-4-12-10(8-11)5-6-18(12)2/h3-8H,1-2H3,(H2,15,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407800

(CHEMBL5291138)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)[C@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C38H53N11O5S/c39-37(40)43-15-5-12-26-33(51)47-28(20-25-11-7-17-55-25)35(53)48-21-24-10-2-1-8-22(24)18-31(48)36(54)49-29-14-4-3-9-23(29)19-30(49)34(52)46-27(32(50)45-26)13-6-16-44-38(41)42/h1-2,7-8,10-11,17,23,26-31H,3-6,9,12-16,18-21H2,(H,45,50)(H,46,52)(H,47,51)(H4,39,40,43)(H4,41,42,44)/t23?,26-,27-,28-,29?,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50986
(MLS000563434 | SMR000232286 | cid_160876)Show InChI InChI=1S/C20H18NO4/c1-22-18-8-13-5-6-21-10-15-12(3-4-17-20(15)25-11-24-17)7-16(21)14(13)9-19(18)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50594961
(CHEMBL5201233)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)C(=O)\C=C\c1ccccc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50587726
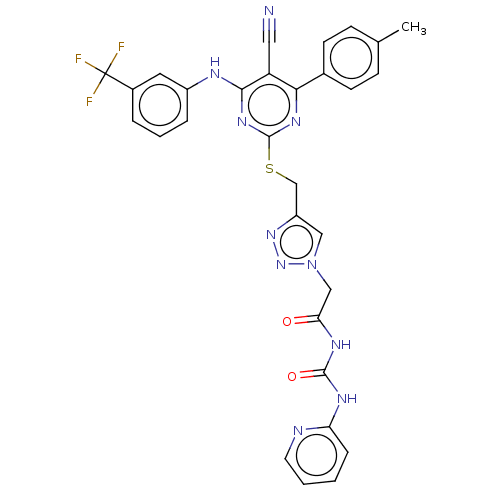
(CHEMBL3401107)Show SMILES Cc1ccc(cc1)-c1nc(SCc2cn(CC(=O)NC(=O)Nc3ccccn3)nn2)nc(Nc2cccc(c2)C(F)(F)F)c1C#N | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM20461
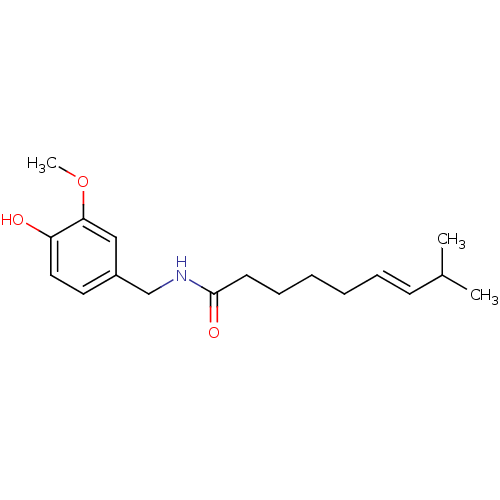
((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...)Show InChI InChI=1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50441978
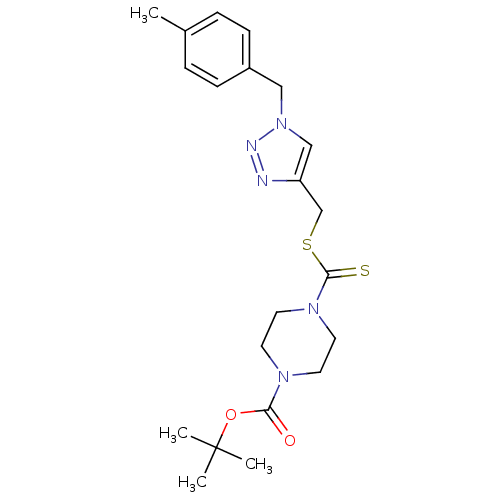
(CHEMBL2334501)Show SMILES Cc1ccc(Cn2cc(CSC(=S)N3CCN(CC3)C(=O)OC(C)(C)C)nn2)cc1 Show InChI InChI=1S/C21H29N5O2S2/c1-16-5-7-17(8-6-16)13-26-14-18(22-23-26)15-30-20(29)25-11-9-24(10-12-25)19(27)28-21(2,3)4/h5-8,14H,9-13,15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50214969

(1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...)Show SMILES [#6]-[#8]-c1c(-[#8])cc2oc3cc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c3c(=O)c2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C24H26O6/c1-12(2)6-8-14-16(25)10-19-21(22(14)27)23(28)20-15(9-7-13(3)4)24(29-5)17(26)11-18(20)30-19/h6-7,10-11,25-27H,8-9H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407799

(CHEMBL5282190)Show SMILES C[C@@H]1NC(=O)CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](CO)NC1=O |r| Show InChI InChI=1S/C35H51N9O7/c1-20-30(47)42-25(19-45)33(50)43-18-23-10-3-2-8-21(23)16-28(43)34(51)44-26-12-5-4-9-22(26)17-27(44)32(49)41-24(11-6-15-39-35(36)37)31(48)38-14-7-13-29(46)40-20/h2-3,8,10,20,22,24-28,45H,4-7,9,11-19H2,1H3,(H,38,48)(H,40,46)(H,41,49)(H,42,47)(H4,36,37,39)/t20-,22?,24-,25-,26?,27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50242173
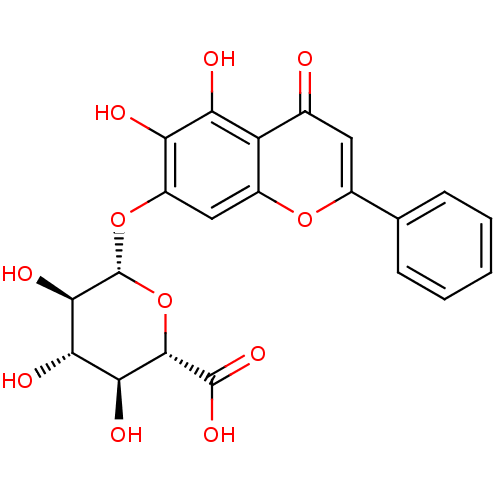
((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...)Show SMILES O[C@H]1[C@H](Oc2cc3oc(cc(=O)c3c(O)c2O)-c2ccccc2)O[C@@H]([C@@H](O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C21H18O11/c22-9-6-10(8-4-2-1-3-5-8)30-11-7-12(14(23)15(24)13(9)11)31-21-18(27)16(25)17(26)19(32-21)20(28)29/h1-7,16-19,21,23-27H,(H,28,29)/t16-,17-,18+,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407801
(CHEMBL5284058)Show SMILES C[C@@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C43H56N10O6/c1-25-41(58)52-24-29-13-3-2-10-26(29)21-36(52)42(59)53-34-16-7-5-12-28(34)22-35(53)40(57)51-32(15-8-19-47-43(44)45)38(55)46-18-9-17-37(54)50-33(39(56)48-25)23-30-20-27-11-4-6-14-31(27)49-30/h2-4,6,10-11,13-14,20,25,28,32-36,49H,5,7-9,12,15-19,21-24H2,1H3,(H,46,55)(H,48,56)(H,50,54)(H,51,57)(H4,44,45,47)/t25-,28?,32-,33-,34?,35-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407720

(CHEMBL5279958)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6]=[#6]P([#8])(=O)[#6]-[#6]-[#6](-[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#6])-[#6])-[#6](-[#6])-[#6])-[#6](-[#8])=O |w:17.17| Show InChI InChI=1S/C33H58N3O7PS/c1-22(2)12-10-13-25(7)14-11-15-26(8)16-19-44(42,43)20-17-28(37)35-29(23(3)4)32(39)36-30(24(5)6)31(38)34-27(33(40)41)18-21-45-9/h12,14,17,20,23-24,26-27,29-30H,10-11,13,15-16,18-19,21H2,1-9H3,(H,34,38)(H,35,37)(H,36,39)(H,40,41)(H,42,43)/b20-17?,25-14+/t26?,27-,29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407803
(CHEMBL5274732)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C65H94N20O13/c66-42(22-10-28-73-62(67)68)54(89)80-46(35-40-18-6-2-7-19-40)56(91)78-43(23-11-29-74-63(69)70)59(94)84-32-15-27-51(84)60(95)83-31-14-26-50(83)58(93)76-37-52(87)77-45(34-39-16-4-1-5-17-39)55(90)81-48(38-86)53(88)49-25-13-33-85(49)65(98)82-47(36-41-20-8-3-9-21-41)57(92)79-44(61(96)97)24-12-30-75-64(71)72/h1-9,16-21,42-51,86H,10-15,22-38,66H2,(H,76,93)(H,77,87)(H,78,91)(H,79,92)(H,80,89)(H,81,90)(H,82,98)(H,96,97)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t42-,43-,44+,45-,46+,47-,48-,49-,50-,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407805
(CHEMBL5274387)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)CCCNC1=O |r| Show InChI InChI=1S/C36H48N8O5S/c37-36(38)40-16-5-12-26-32(46)39-15-6-14-31(45)41-27(20-25-11-7-17-50-25)34(48)43-21-24-10-2-1-8-22(24)18-30(43)35(49)44-28-13-4-3-9-23(28)19-29(44)33(47)42-26/h1-2,7-8,10-11,17,23,26-30H,3-6,9,12-16,18-21H2,(H,39,46)(H,41,45)(H,42,47)(H4,37,38,40)/t23?,26-,27-,28?,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407807
(CHEMBL5270254)Show SMILES C[C@@H]1NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C30H42N8O5/c1-17-28(42)37-16-20-9-3-2-7-18(20)13-24(37)29(43)38-22-11-5-4-8-19(22)14-23(38)27(41)36-21(10-6-12-33-30(31)32)26(40)34-15-25(39)35-17/h2-3,7,9,17,19,21-24H,4-6,8,10-16H2,1H3,(H,34,40)(H,35,39)(H,36,41)(H4,31,32,33)/t17-,19?,21-,22?,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against calcitonin gene related peptide receptor determined by measuring the formation of cyclic AMP in SK-N-MC cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407798

(CHEMBL3134309)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C34H44N8O5S/c35-34(36)37-13-5-11-24-30(44)38-18-29(43)39-25(17-23-10-6-14-48-23)32(46)41-19-22-9-2-1-7-20(22)15-28(41)33(47)42-26-12-4-3-8-21(26)16-27(42)31(45)40-24/h1-2,6-7,9-10,14,21,24-28H,3-5,8,11-13,15-19H2,(H,38,44)(H,39,43)(H,40,45)(H4,35,36,37)/t21?,24-,25-,26?,27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407802
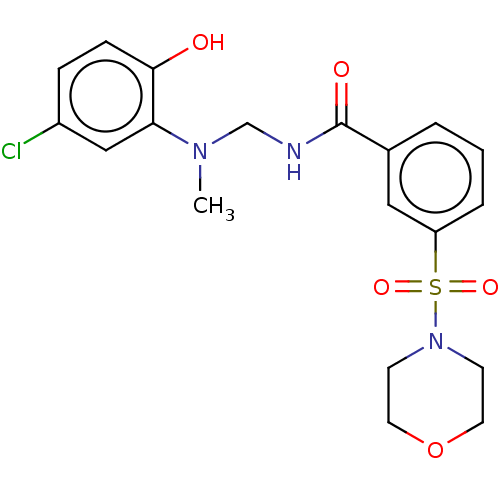
(CHEMBL5271269)Show SMILES NCCCC[C@@H]1N[C@@H](Cc2cccs2)C(=O)N2Cc3ccccc3C[C@@H]2C(=O)N2C3CCCCC3C[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1=O |r| Show InChI InChI=1S/C38H53N9O5S/c39-16-6-5-13-27-33(48)45-34(49)28(14-7-17-42-38(40)41)44-35(50)31-20-24-10-3-4-15-30(24)47(31)37(52)32-19-23-9-1-2-11-25(23)22-46(32)36(51)29(43-27)21-26-12-8-18-53-26/h1-2,8-9,11-12,18,24,27-32,43H,3-7,10,13-17,19-22,39H2,(H,44,50)(H4,40,41,42)(H,45,48,49)/t24?,27-,28-,29-,30?,31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407808
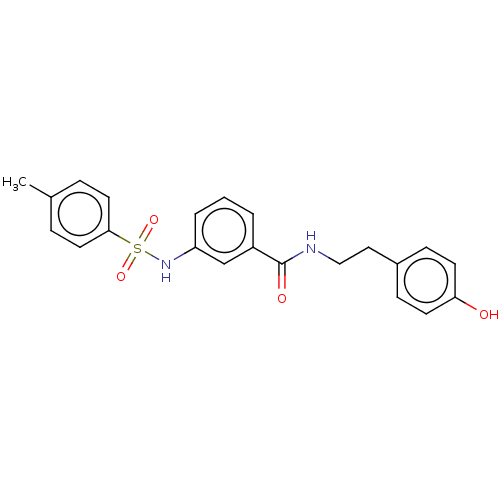
(CHEMBL5283677)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](CO)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)CCCNC1=O |r| Show InChI InChI=1S/C42H55N9O7/c43-42(44)46-18-7-14-30-37(54)45-17-8-16-36(53)49-22-28-12-3-1-9-25(28)19-33(49)38(55)48-31(24-52)40(57)50-23-29-13-4-2-10-26(29)20-35(50)41(58)51-32-15-6-5-11-27(32)21-34(51)39(56)47-30/h1-4,9-10,12-13,27,30-35,52H,5-8,11,14-24H2,(H,45,54)(H,47,56)(H,48,55)(H4,43,44,46)/t27?,30-,31-,32?,33-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against calcitonin gene related peptide receptor determined by measuring the formation of cyclic AMP in SK-N-MC cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158878
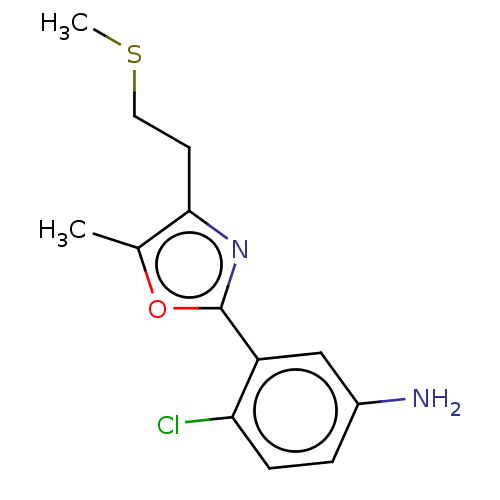
(CHEMBL3785551)Show InChI InChI=1S/C13H15ClN2OS/c1-8-12(5-6-18-2)16-13(17-8)10-7-9(15)3-4-11(10)14/h3-4,7H,5-6,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407719

(CHEMBL5281939)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6]-[#8]P([#8])(=O)[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](-[#8])=O Show InChI InChI=1S/C31H54N3O9P/c1-20(2)11-9-12-23(7)13-10-14-24(8)15-16-44(41,42)43-19-27(36)34-28(22(5)6)30(38)32-25(17-21(3)4)29(37)33-26(18-35)31(39)40/h11,13,15,21-22,25-26,28,35H,9-10,12,14,16-19H2,1-8H3,(H,32,38)(H,33,37)(H,34,36)(H,39,40)(H,41,42)/b23-13+,24-15+/t25-,26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407797

(CHEMBL5290659)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C36H46N8O5/c37-36(38)39-16-8-14-26-32(46)40-20-31(45)41-27(17-22-9-2-1-3-10-22)34(48)43-21-25-13-5-4-11-23(25)18-30(43)35(49)44-28-15-7-6-12-24(28)19-29(44)33(47)42-26/h1-5,9-11,13,24,26-30H,6-8,12,14-21H2,(H,40,46)(H,41,45)(H,42,47)(H4,37,38,39)/t24?,26-,27-,28?,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50594961

(CHEMBL5201233)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)C(=O)\C=C\c1ccccc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50594961

(CHEMBL5201233)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)C(=S)SCC(=O)Nc1ccc(cc1)C(=O)\C=C\c1ccccc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50445351

(NAMOLINE)Show SMILES [O-][N+](=O)c1ccc2oc(c(Cl)c(=O)c2c1)C(F)(F)F Show InChI InChI=1S/C10H3ClF3NO4/c11-7-8(16)5-3-4(15(17)18)1-2-6(5)19-9(7)10(12,13)14/h1-3H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50407807

(CHEMBL5270254)Show SMILES C[C@@H]1NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C30H42N8O5/c1-17-28(42)37-16-20-9-3-2-7-18(20)13-24(37)29(43)38-22-11-5-4-8-19(22)14-23(38)27(41)36-21(10-6-12-33-30(31)32)26(40)34-15-25(39)35-17/h2-3,7,9,17,19,21-24H,4-6,8,10-16H2,1H3,(H,34,40)(H,35,39)(H,36,41)(H4,31,32,33)/t17-,19?,21-,22?,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50407802

(CHEMBL5271269)Show SMILES NCCCC[C@@H]1N[C@@H](Cc2cccs2)C(=O)N2Cc3ccccc3C[C@@H]2C(=O)N2C3CCCCC3C[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1=O |r| Show InChI InChI=1S/C38H53N9O5S/c39-16-6-5-13-27-33(48)45-34(49)28(14-7-17-42-38(40)41)44-35(50)31-20-24-10-3-4-15-30(24)47(31)37(52)32-19-23-9-1-2-11-25(23)22-46(32)36(51)29(43-27)21-26-12-8-18-53-26/h1-2,8-9,11-12,18,24,27-32,43H,3-7,10,13-17,19-22,39H2,(H,44,50)(H4,40,41,42)(H,45,48,49)/t24?,27-,28-,29-,30?,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50407801

(CHEMBL5284058)Show SMILES C[C@@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C43H56N10O6/c1-25-41(58)52-24-29-13-3-2-10-26(29)21-36(52)42(59)53-34-16-7-5-12-28(34)22-35(53)40(57)51-32(15-8-19-47-43(44)45)38(55)46-18-9-17-37(54)50-33(39(56)48-25)23-30-20-27-11-4-6-14-31(27)49-30/h2-4,6,10-11,13-14,20,25,28,32-36,49H,5,7-9,12,15-19,21-24H2,1H3,(H,46,55)(H,48,56)(H,50,54)(H,51,57)(H4,44,45,47)/t25-,28?,32-,33-,34?,35-,36+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against calcitonin gene related peptide receptor determined by measuring the formation of cyclic AMP in SK-N-MC cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50407805

(CHEMBL5274387)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)CCCNC1=O |r| Show InChI InChI=1S/C36H48N8O5S/c37-36(38)40-16-5-12-26-32(46)39-15-6-14-31(45)41-27(20-25-11-7-17-50-25)34(48)43-21-24-10-2-1-8-22(24)18-30(43)35(49)44-28-13-4-3-9-23(28)19-29(44)33(47)42-26/h1-2,7-8,10-11,17,23,26-30H,3-6,9,12-16,18-21H2,(H,39,46)(H,41,45)(H,42,47)(H4,37,38,40)/t23?,26-,27-,28?,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50407803

(CHEMBL5274732)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C65H94N20O13/c66-42(22-10-28-73-62(67)68)54(89)80-46(35-40-18-6-2-7-19-40)56(91)78-43(23-11-29-74-63(69)70)59(94)84-32-15-27-51(84)60(95)83-31-14-26-50(83)58(93)76-37-52(87)77-45(34-39-16-4-1-5-17-39)55(90)81-48(38-86)53(88)49-25-13-33-85(49)65(98)82-47(36-41-20-8-3-9-21-41)57(92)79-44(61(96)97)24-12-30-75-64(71)72/h1-9,16-21,42-51,86H,10-15,22-38,66H2,(H,76,93)(H,77,87)(H,78,91)(H,79,92)(H,80,89)(H,81,90)(H,82,98)(H,96,97)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t42-,43-,44+,45-,46+,47-,48-,49-,50-,51-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50407808

(CHEMBL5283677)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](CO)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)CCCNC1=O |r| Show InChI InChI=1S/C42H55N9O7/c43-42(44)46-18-7-14-30-37(54)45-17-8-16-36(53)49-22-28-12-3-1-9-25(28)19-33(49)38(55)48-31(24-52)40(57)50-23-29-13-4-2-10-26(29)20-35(50)41(58)51-32-15-6-5-11-27(32)21-34(51)39(56)47-30/h1-4,9-10,12-13,27,30-35,52H,5-8,11,14-24H2,(H,45,54)(H,47,56)(H,48,55)(H4,43,44,46)/t27?,30-,31-,32?,33-,34-,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50407803

(CHEMBL5274732)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C65H94N20O13/c66-42(22-10-28-73-62(67)68)54(89)80-46(35-40-18-6-2-7-19-40)56(91)78-43(23-11-29-74-63(69)70)59(94)84-32-15-27-51(84)60(95)83-31-14-26-50(83)58(93)76-37-52(87)77-45(34-39-16-4-1-5-17-39)55(90)81-48(38-86)53(88)49-25-13-33-85(49)65(98)82-47(36-41-20-8-3-9-21-41)57(92)79-44(61(96)97)24-12-30-75-64(71)72/h1-9,16-21,42-51,86H,10-15,22-38,66H2,(H,76,93)(H,77,87)(H,78,91)(H,79,92)(H,80,89)(H,81,90)(H,82,98)(H,96,97)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t42-,43-,44+,45-,46+,47-,48-,49-,50-,51-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against calcitonin gene related peptide receptor determined by measuring the formation of cyclic AMP in SK-N-MC cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50407801

(CHEMBL5284058)Show SMILES C[C@@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C43H56N10O6/c1-25-41(58)52-24-29-13-3-2-10-26(29)21-36(52)42(59)53-34-16-7-5-12-28(34)22-35(53)40(57)51-32(15-8-19-47-43(44)45)38(55)46-18-9-17-37(54)50-33(39(56)48-25)23-30-20-27-11-4-6-14-31(27)49-30/h2-4,6,10-11,13-14,20,25,28,32-36,49H,5,7-9,12,15-19,21-24H2,1H3,(H,46,55)(H,48,56)(H,50,54)(H,51,57)(H4,44,45,47)/t25-,28?,32-,33-,34?,35-,36+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50407802

(CHEMBL5271269)Show SMILES NCCCC[C@@H]1N[C@@H](Cc2cccs2)C(=O)N2Cc3ccccc3C[C@@H]2C(=O)N2C3CCCCC3C[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1=O |r| Show InChI InChI=1S/C38H53N9O5S/c39-16-6-5-13-27-33(48)45-34(49)28(14-7-17-42-38(40)41)44-35(50)31-20-24-10-3-4-15-30(24)47(31)37(52)32-19-23-9-1-2-11-25(23)22-46(32)36(51)29(43-27)21-26-12-8-18-53-26/h1-2,8-9,11-12,18,24,27-32,43H,3-7,10,13-17,19-22,39H2,(H,44,50)(H4,40,41,42)(H,45,48,49)/t24?,27-,28-,29-,30?,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against calcitonin gene related peptide receptor determined by measuring the formation of cyclic AMP in SK-N-MC cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50407805

(CHEMBL5274387)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)CCCNC1=O |r| Show InChI InChI=1S/C36H48N8O5S/c37-36(38)40-16-5-12-26-32(46)39-15-6-14-31(45)41-27(20-25-11-7-17-50-25)34(48)43-21-24-10-2-1-8-22(24)18-30(43)35(49)44-28-13-4-3-9-23(28)19-29(44)33(47)42-26/h1-2,7-8,10-11,17,23,26-30H,3-6,9,12-16,18-21H2,(H,39,46)(H,41,45)(H,42,47)(H4,37,38,40)/t23?,26-,27-,28?,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50407807

(CHEMBL5270254)Show SMILES C[C@@H]1NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C30H42N8O5/c1-17-28(42)37-16-20-9-3-2-7-18(20)13-24(37)29(43)38-22-11-5-4-8-19(22)14-23(38)27(41)36-21(10-6-12-33-30(31)32)26(40)34-15-25(39)35-17/h2-3,7,9,17,19,21-24H,4-6,8,10-16H2,1H3,(H,34,40)(H,35,39)(H,36,41)(H4,31,32,33)/t17-,19?,21-,22?,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50407808

(CHEMBL5283677)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](CO)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)CCCNC1=O |r| Show InChI InChI=1S/C42H55N9O7/c43-42(44)46-18-7-14-30-37(54)45-17-8-16-36(53)49-22-28-12-3-1-9-25(28)19-33(49)38(55)48-31(24-52)40(57)50-23-29-13-4-2-10-26(29)20-35(50)41(58)51-32-15-6-5-11-27(32)21-34(51)39(56)47-30/h1-4,9-10,12-13,27,30-35,52H,5-8,11,14-24H2,(H,45,54)(H,47,56)(H,48,55)(H4,43,44,46)/t27?,30-,31-,32?,33-,34-,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50507295
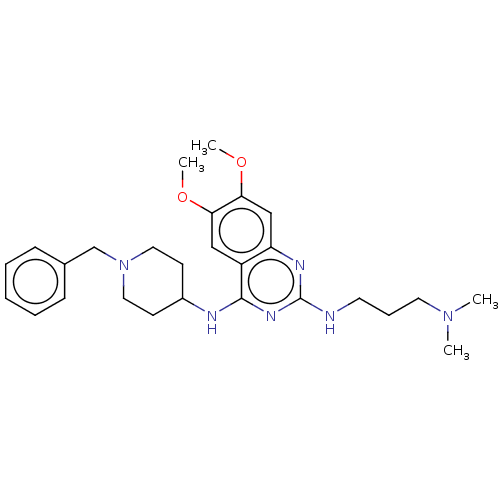
(CHEMBL1232432)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC Show InChI InChI=1S/C27H38N6O2/c1-32(2)14-8-13-28-27-30-23-18-25(35-4)24(34-3)17-22(23)26(31-27)29-21-11-15-33(16-12-21)19-20-9-6-5-7-10-20/h5-7,9-10,17-18,21H,8,11-16,19H2,1-4H3,(H2,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50594962

(CHEMBL5177178)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data