

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50261701 (2-Methyl-c-5-[4-(5-methyl-2-phenyloxazol-4-ylmetho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.09 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50261701 (2-Methyl-c-5-[4-(5-methyl-2-phenyloxazol-4-ylmetho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 96 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50261879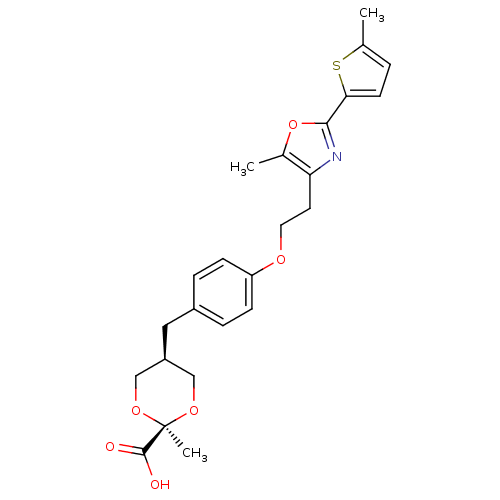 (2-Methyl-c-5-{4-[2-(5-methyl-2-(5-methylthiophen-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50261878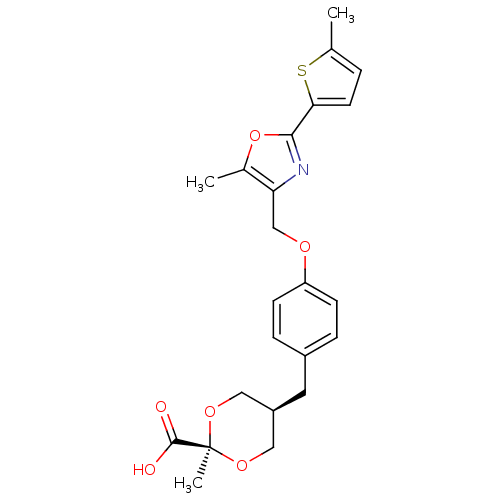 (2-Methyl-c-5-{4-[5-methyl-2-(5-methylthiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50261703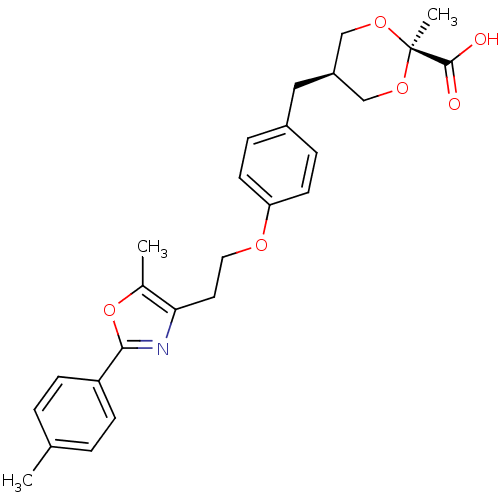 (2-Methyl-c-5-{4-[2-(5-methyl-2-(4-methylphenyl)oxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50261702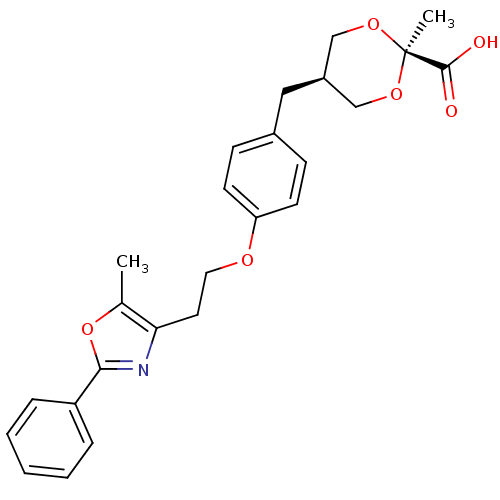 (2-Methyl-c-5-[4-{2-(5-methyl-2-phenyloxazol-4-yl)e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.272 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50256109 ((2s,5s)-2-methyl-5-(4-((5-methyl-2-p-tolyloxazol-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50261880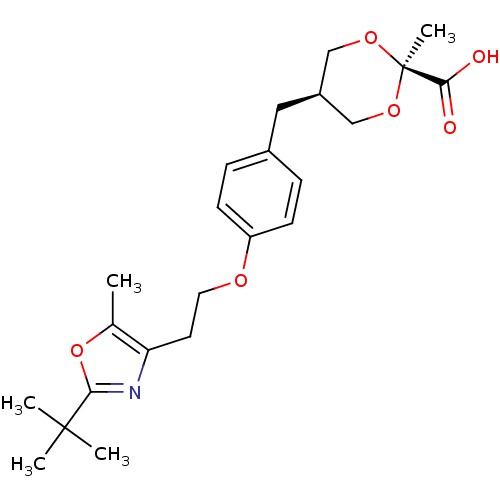 (CHEMBL512789 | c-5-[4-{2-(2-tert-Butyl-5-methyloxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50261879 (2-Methyl-c-5-{4-[2-(5-methyl-2-(5-methylthiophen-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 239 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50261878 (2-Methyl-c-5-{4-[5-methyl-2-(5-methylthiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19.8 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50261703 (2-Methyl-c-5-{4-[2-(5-methyl-2-(4-methylphenyl)oxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50261702 (2-Methyl-c-5-[4-{2-(5-methyl-2-phenyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50256109 ((2s,5s)-2-methyl-5-(4-((5-methyl-2-p-tolyloxazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM24566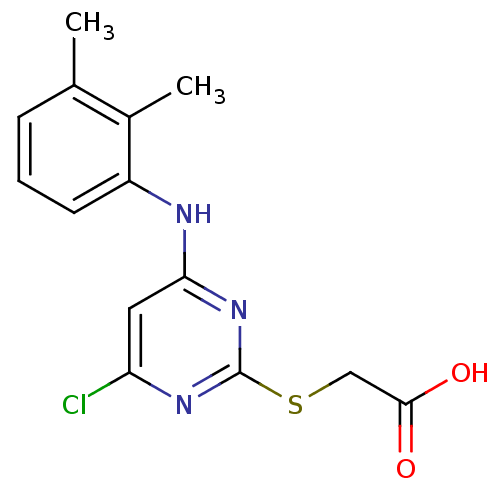 (2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50261880 (CHEMBL512789 | c-5-[4-{2-(2-tert-Butyl-5-methyloxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa... | Bioorg Med Chem 16: 7117-27 (2008) Article DOI: 10.1016/j.bmc.2008.06.050 BindingDB Entry DOI: 10.7270/Q2K93792 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||