

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50005463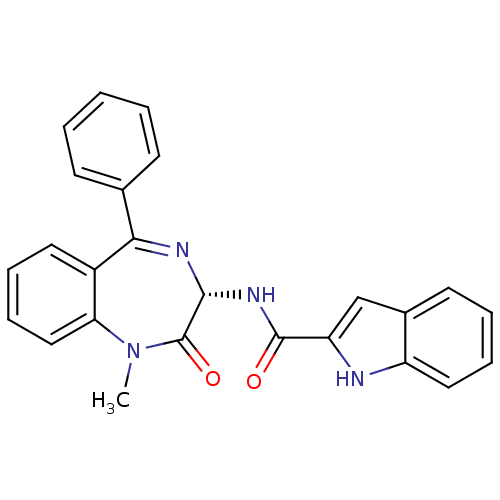 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281730 (4-Chloro-N-((S)-1-methyl-2-oxo-5-phenyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50006878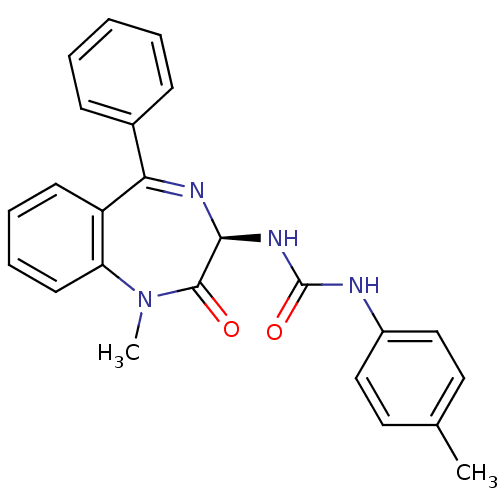 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.5 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281732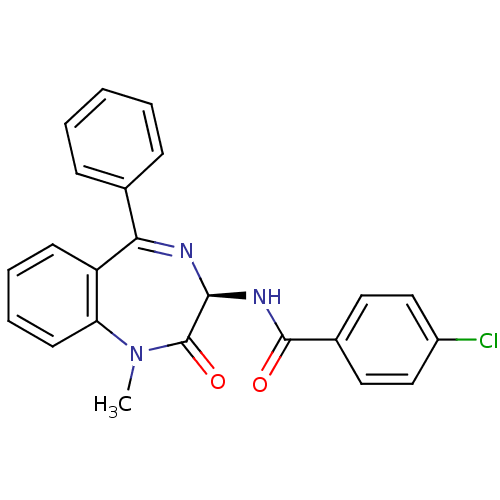 (4-Chloro-N-((R)-1-methyl-2-oxo-5-phenyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061220 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060321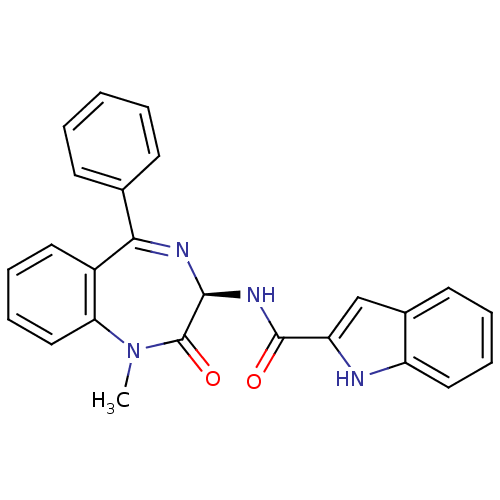 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281729 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carbothi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [3H]-L-364,718 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50092155 ((4S,5R)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in mouse brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50092155 ((4S,5R)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 6.5 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in mouse brain at a pH of 7.4 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281729 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carbothi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [3H]-L-364,718 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50061220 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 6.5 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 6.5 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50092157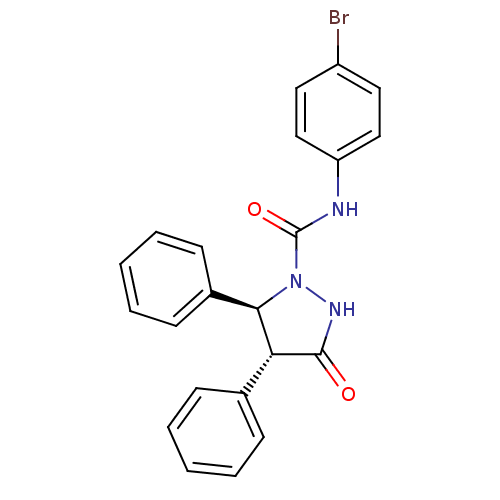 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.4 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in mouse brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50281731 ((4S,5R)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carbothi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.4 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in mouse brain at a pH of 7.4 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281731 ((4S,5R)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carbothi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [3H]-L-364,718 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50281729 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carbothi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 880 | n/a | n/a | n/a | n/a | 7.4 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in mouse brain at a pH of 7.4 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50281729 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carbothi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in mouse brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50281730 (4-Chloro-N-((S)-1-methyl-2-oxo-5-phenyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092157 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [3H]-L-364,718 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50281732 (4-Chloro-N-((R)-1-methyl-2-oxo-5-phenyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092155 ((4S,5R)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [3H]-L-364,718 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092155 ((4S,5R)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [3H]-L-364,718 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||