 Found 68 hits Enz. Inhib. hit(s) with all data for entry = 50033243
Found 68 hits Enz. Inhib. hit(s) with all data for entry = 50033243 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
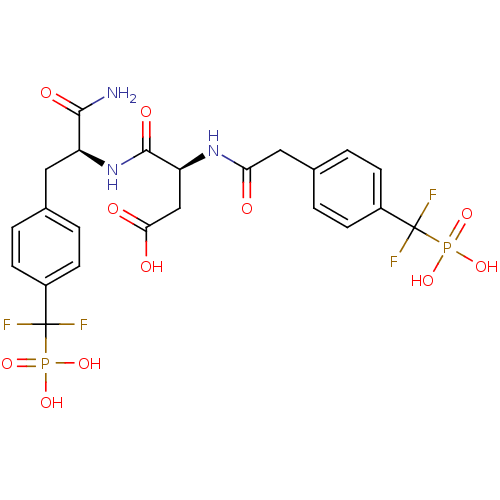
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50341987
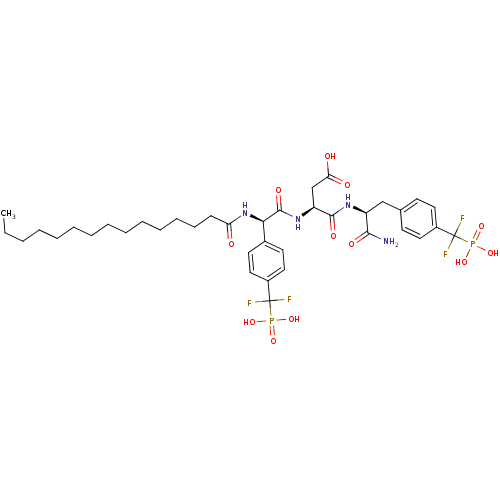
((S)-4-((S)-1-amino-3-(4-(difluoro(phosphono)methyl...)Show SMILES CCCCCCCCCCCCCCC(=O)N[C@@H](C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(N)=O)c1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C38H54F4N4O12P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-31(47)46-33(26-17-21-28(22-18-26)38(41,42)60(56,57)58)36(52)45-30(24-32(48)49)35(51)44-29(34(43)50)23-25-15-19-27(20-16-25)37(39,40)59(53,54)55/h15-22,29-30,33H,2-14,23-24H2,1H3,(H2,43,50)(H,44,51)(H,45,52)(H,46,47)(H,48,49)(H2,53,54,55)(H2,56,57,58)/t29-,30-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25A |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25C |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341982
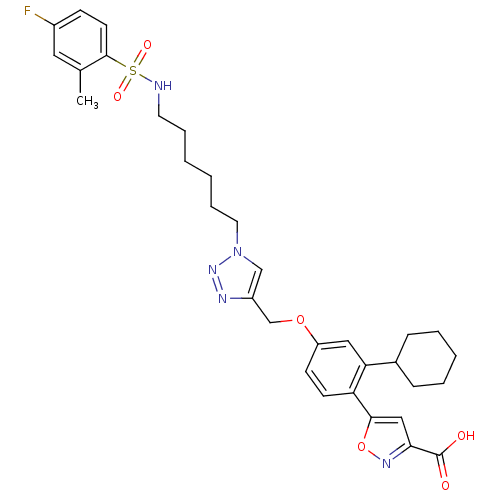
(5-(2-cyclohexyl-4-((1-(6-(4-fluoro-2-methylphenyls...)Show SMILES Cc1cc(F)ccc1S(=O)(=O)NCCCCCCn1cc(COc2ccc(-c3cc(no3)C(O)=O)c(c2)C2CCCCC2)nn1 Show InChI InChI=1S/C32H38FN5O6S/c1-22-17-24(33)11-14-31(22)45(41,42)34-15-7-2-3-8-16-38-20-25(35-37-38)21-43-26-12-13-27(30-19-29(32(39)40)36-44-30)28(18-26)23-9-5-4-6-10-23/h11-14,17-20,23,34H,2-10,15-16,21H2,1H3,(H,39,40) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341985
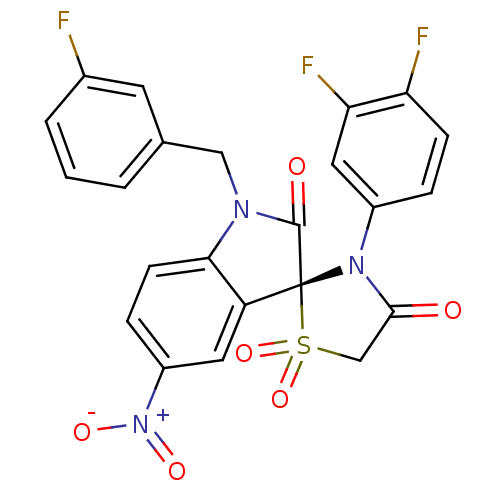
(CHEMBL1765366)Show SMILES [O-][N+](=O)c1ccc2N(Cc3cccc(F)c3)C(=O)[C@@]3(N(C(=O)CS3(=O)=O)c3ccc(F)c(F)c3)c2c1 |r| Show InChI InChI=1S/C23H14F3N3O6S/c24-14-3-1-2-13(8-14)11-27-20-7-5-16(29(32)33)9-17(20)23(22(27)31)28(21(30)12-36(23,34)35)15-4-6-18(25)19(26)10-15/h1-10H,11-12H2/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50342004

(4-(2-(3-(4-nitrophenyl)-5-oxo-1-phenyl-1H-pyrazol-...)Show SMILES OS(=O)(=O)c1ccc(cc1)N=Nc1c([nH]n(-c2ccccc2)c1=O)-c1ccc(cc1)[N+]([O-])=O |w:10.10| Show InChI InChI=1S/C21H15N5O6S/c27-21-20(23-22-15-8-12-18(13-9-15)33(30,31)32)19(14-6-10-17(11-7-14)26(28)29)24-25(21)16-4-2-1-3-5-16/h1-13,24H,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of SHP-2 |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Low molecular weight protein-tyrosine phosphatase A
(Mycobacterium tuberculosis) | BDBM50341977
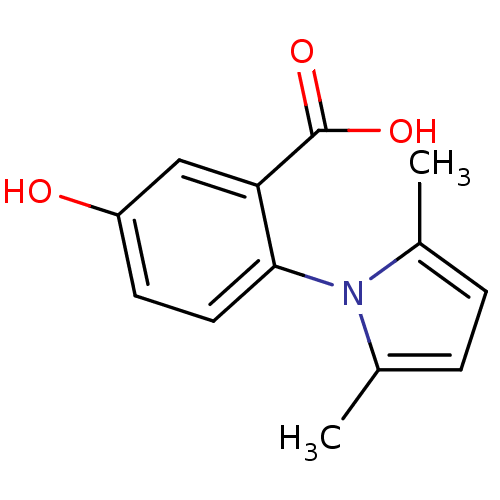
(2-(2,5-dimethyl-1H-pyrrol-1-yl)-5-hydroxybenzoic a...)Show SMILES Cc1ccc(C)n1-c1ccc(O)cc1C(O)=O |(5.57,-.15,;4.1,-.62,;3.62,-2.09,;2.08,-2.09,;1.61,-.62,;.14,-.15,;2.86,.28,;2.85,1.82,;1.51,2.59,;1.51,4.14,;2.84,4.91,;2.84,6.45,;4.18,4.14,;4.18,2.59,;5.66,2.2,;6.75,3.29,;6.06,.71,)| Show InChI InChI=1S/C13H13NO3/c1-8-3-4-9(2)14(8)12-6-5-10(15)7-11(12)13(16)17/h3-7,15H,1-2H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpA |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50341977

(2-(2,5-dimethyl-1H-pyrrol-1-yl)-5-hydroxybenzoic a...)Show SMILES Cc1ccc(C)n1-c1ccc(O)cc1C(O)=O |(5.57,-.15,;4.1,-.62,;3.62,-2.09,;2.08,-2.09,;1.61,-.62,;.14,-.15,;2.86,.28,;2.85,1.82,;1.51,2.59,;1.51,4.14,;2.84,4.91,;2.84,6.45,;4.18,4.14,;4.18,2.59,;5.66,2.2,;6.75,3.29,;6.06,.71,)| Show InChI InChI=1S/C13H13NO3/c1-8-3-4-9(2)14(8)12-6-5-10(15)7-11(12)13(16)17/h3-7,15H,1-2H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50341988
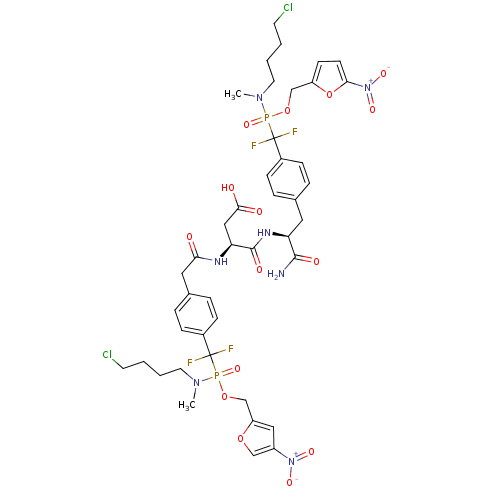
((3S)-4-((2S)-1-amino-3-(4-((((4-chlorobutyl)(methy...)Show SMILES CN(CCCCCl)P(=O)(OCc1ccc(o1)[N+]([O-])=O)C(F)(F)c1ccc(C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)Cc2ccc(cc2)C(F)(F)P(=O)(OCc2cc(co2)[N+]([O-])=O)N(C)CCCCCl)C(N)=O)cc1 |r| Show InChI InChI=1S/C43H51Cl2F4N7O15P2/c1-53(19-5-3-17-44)72(66,69-26-33-15-16-38(71-33)56(64)65)42(46,47)30-11-7-28(8-12-30)21-35(40(50)60)52-41(61)36(24-39(58)59)51-37(57)22-29-9-13-31(14-10-29)43(48,49)73(67,54(2)20-6-4-18-45)70-27-34-23-32(25-68-34)55(62)63/h7-16,23,25,35-36H,3-6,17-22,24,26-27H2,1-2H3,(H2,50,60)(H,51,57)(H,52,61)(H,58,59)/t35-,36-,72?,73?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50208827

(2,3-bis(2-hydroxyethylthio)naphthalene-1,4-dione |...)Show InChI InChI=1S/C14H14O4S2/c15-5-7-19-13-11(17)9-3-1-2-4-10(9)12(18)14(13)20-8-6-16/h1-4,15-16H,5-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25A |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50341990
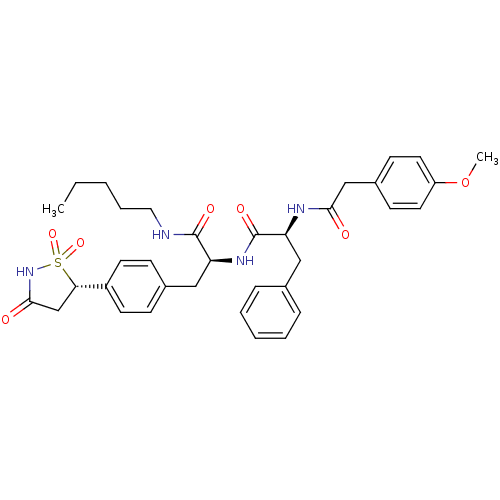
((S)-2-[2-(4-Methoxy-phenyl)-acetylamino]-N-{(S)-1-...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(cc1)[C@@H]1CC(=O)NS1(=O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(OC)cc1 |r| Show InChI InChI=1S/C35H42N4O7S/c1-3-4-8-19-36-34(42)29(21-25-11-15-27(16-12-25)31-23-33(41)39-47(31,44)45)38-35(43)30(20-24-9-6-5-7-10-24)37-32(40)22-26-13-17-28(46-2)18-14-26/h5-7,9-18,29-31H,3-4,8,19-23H2,1-2H3,(H,36,42)(H,37,40)(H,38,43)(H,39,41)/t29-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50208827

(2,3-bis(2-hydroxyethylthio)naphthalene-1,4-dione |...)Show InChI InChI=1S/C14H14O4S2/c15-5-7-19-13-11(17)9-3-1-2-4-10(9)12(18)14(13)20-8-6-16/h1-4,15-16H,5-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25C |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50303174

(2-(5-((3-(4-(2-fluorobenzyloxy)phenyl)-1-phenyl-1H...)Show SMILES OS(=O)(=O)CCN1C(=S)S\C(=C/c2cn(nc2-c2ccc(OCc3ccccc3F)cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H22FN3O5S3/c29-24-9-5-4-6-20(24)18-37-23-12-10-19(11-13-23)26-21(17-32(30-26)22-7-2-1-3-8-22)16-25-27(33)31(28(38)39-25)14-15-40(34,35)36/h1-13,16-17H,14-15,18H2,(H,34,35,36)/b25-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308840
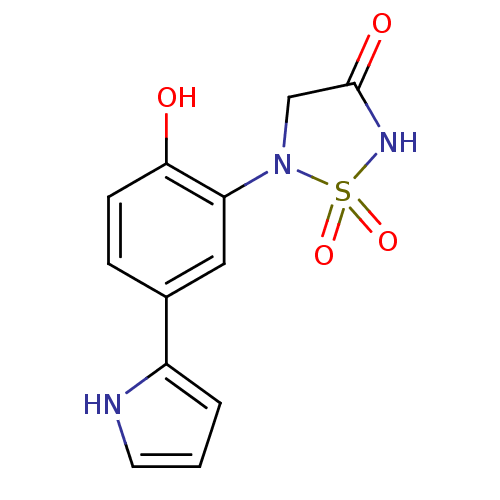
(5-[2-Hydroxy-5-(1H-pyrrol-2-yl)-phenyl]-1,1-dioxo-...)Show InChI InChI=1S/C12H11N3O4S/c16-11-4-3-8(9-2-1-5-13-9)6-10(11)15-7-12(17)14-20(15,18)19/h1-6,13,16H,7H2,(H,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50239833
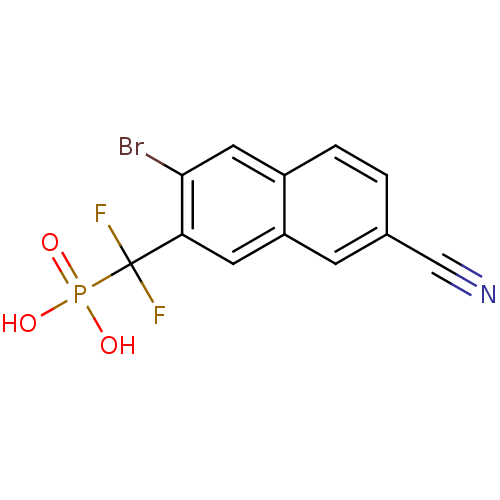
((3-bromo-7-cyanonaphthalen-2-yl)difluoromethylphos...)Show InChI InChI=1S/C12H7BrF2NO3P/c13-11-5-8-2-1-7(6-16)3-9(8)4-10(11)12(14,15)20(17,18)19/h1-5H,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50208827

(2,3-bis(2-hydroxyethylthio)naphthalene-1,4-dione |...)Show InChI InChI=1S/C14H14O4S2/c15-5-7-19-13-11(17)9-3-1-2-4-10(9)12(18)14(13)20-8-6-16/h1-4,15-16H,5-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308839
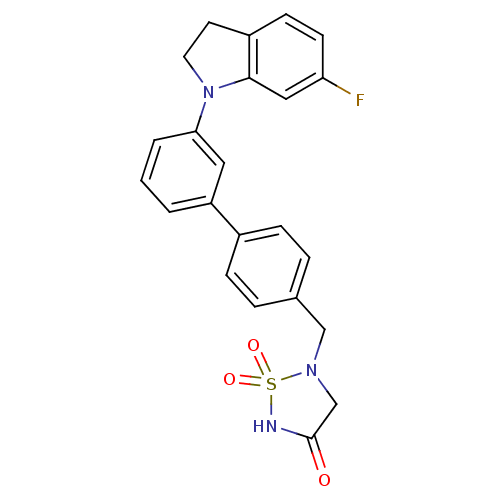
(5-[3'-(6-Fluoro-2,3-dihydro-indol-1-yl)-biphenyl-4...)Show SMILES Fc1ccc2CCN(c2c1)c1cccc(c1)-c1ccc(CN2CC(=O)NS2(=O)=O)cc1 Show InChI InChI=1S/C23H20FN3O3S/c24-20-9-8-18-10-11-27(22(18)13-20)21-3-1-2-19(12-21)17-6-4-16(5-7-17)14-26-15-23(28)25-31(26,29)30/h1-9,12-13H,10-11,14-15H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308846
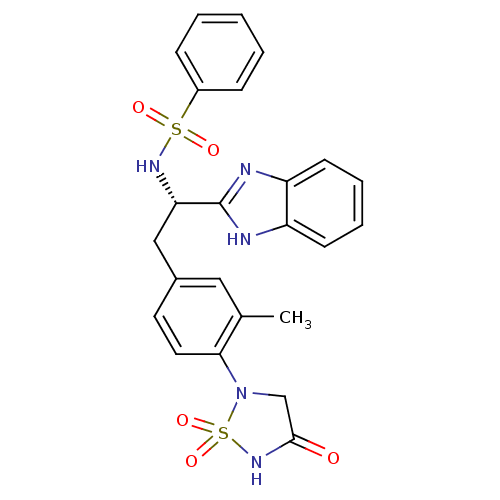
(CHEMBL592245 | N-{(S)-1-(1H-Benzoimidazol-2-yl)-2-...)Show SMILES Cc1cc(C[C@H](NS(=O)(=O)c2ccccc2)c2nc3ccccc3[nH]2)ccc1N1CC(=O)NS1(=O)=O |r| Show InChI InChI=1S/C24H23N5O5S2/c1-16-13-17(11-12-22(16)29-15-23(30)28-36(29,33)34)14-21(24-25-19-9-5-6-10-20(19)26-24)27-35(31,32)18-7-3-2-4-8-18/h2-13,21,27H,14-15H2,1H3,(H,25,26)(H,28,30)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50341995

(5-(2-(dimethylamino)ethylamino)-2,6-dimethylbenzo[...)Show InChI InChI=1S/C13H17N3O2S/c1-7-9(14-5-6-16(3)4)12(18)10-13(11(7)17)19-8(2)15-10/h5,17-18H,6H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25C |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50341993
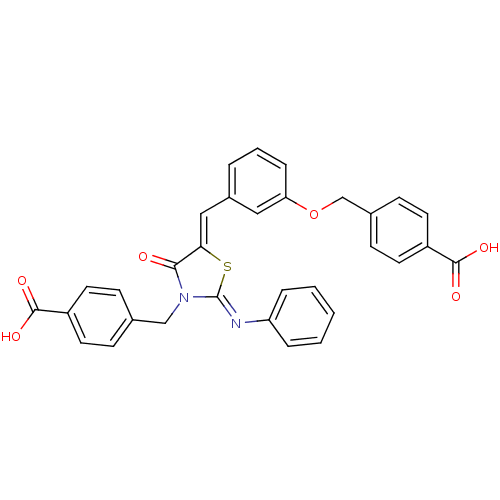
(4-((3-((3-(4-carboxybenzyl)-4-oxo-2-(phenylimino)t...)Show SMILES OC(=O)c1ccc(COc2cccc(\C=C3/S\C(=N/c4ccccc4)N(Cc4ccc(cc4)C(O)=O)C3=O)c2)cc1 Show InChI InChI=1S/C32H24N2O6S/c35-29-28(18-23-5-4-8-27(17-23)40-20-22-11-15-25(16-12-22)31(38)39)41-32(33-26-6-2-1-3-7-26)34(29)19-21-9-13-24(14-10-21)30(36)37/h1-18H,19-20H2,(H,36,37)(H,38,39)/b28-18-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM26104
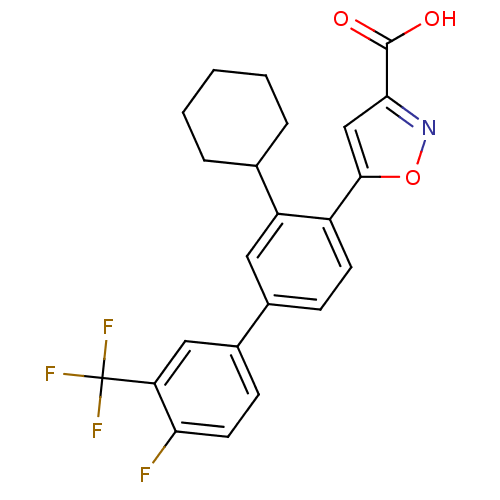
(5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phe...)Show SMILES OC(=O)c1cc(on1)-c1ccc(cc1C1CCCCC1)-c1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C23H19F4NO3/c24-19-9-7-15(11-18(19)23(25,26)27)14-6-8-16(21-12-20(22(29)30)28-31-21)17(10-14)13-4-2-1-3-5-13/h6-13H,1-5H2,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50341994
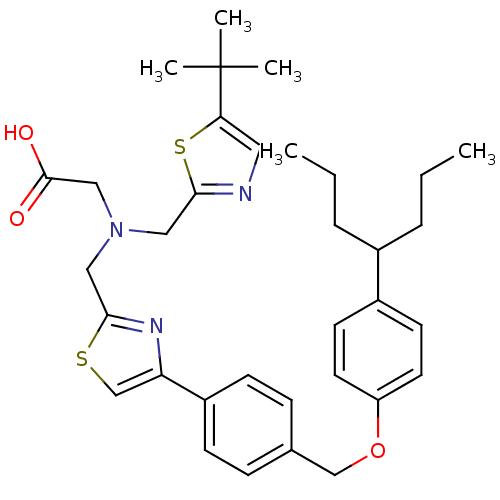
(2-(((5-tert-butylthiazol-2-yl)methyl)((4-(4-((4-(h...)Show SMILES CCCC(CCC)c1ccc(OCc2ccc(cc2)-c2csc(CN(CC(O)=O)Cc3ncc(s3)C(C)(C)C)n2)cc1 Show InChI InChI=1S/C34H43N3O3S2/c1-6-8-25(9-7-2)26-14-16-28(17-15-26)40-22-24-10-12-27(13-11-24)29-23-41-32(36-29)20-37(21-33(38)39)19-31-35-18-30(42-31)34(3,4)5/h10-18,23,25H,6-9,19-22H2,1-5H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50341995

(5-(2-(dimethylamino)ethylamino)-2,6-dimethylbenzo[...)Show InChI InChI=1S/C13H17N3O2S/c1-7-9(14-5-6-16(3)4)12(18)10-13(11(7)17)19-8(2)15-10/h5,17-18H,6H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50341995

(5-(2-(dimethylamino)ethylamino)-2,6-dimethylbenzo[...)Show InChI InChI=1S/C13H17N3O2S/c1-7-9(14-5-6-16(3)4)12(18)10-13(11(7)17)19-8(2)15-10/h5,17-18H,6H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25A |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50342003
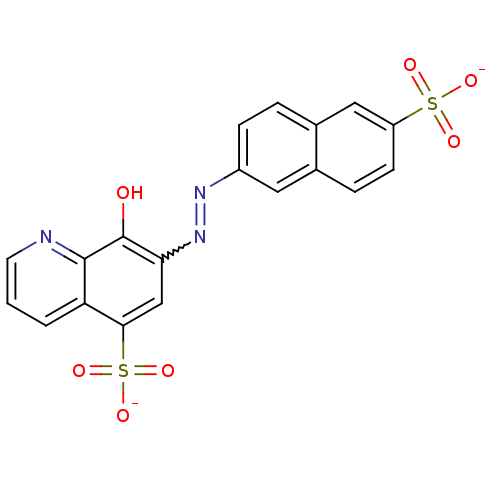
(CHEMBL501993 | NSC-87877 | disodium 8-hydroxy-7-[(...)Show SMILES Oc1c(cc(c2cccnc12)S([O-])(=O)=O)N=Nc1ccc2cc(ccc2c1)S([O-])(=O)=O |w:15.16| Show InChI InChI=1S/C19H13N3O7S2/c23-19-16(10-17(31(27,28)29)15-2-1-7-20-18(15)19)22-21-13-5-3-12-9-14(30(24,25)26)6-4-11(12)8-13/h1-10,23H,(H,24,25,26)(H,27,28,29)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of SHP-2 |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341985

(CHEMBL1765366)Show SMILES [O-][N+](=O)c1ccc2N(Cc3cccc(F)c3)C(=O)[C@@]3(N(C(=O)CS3(=O)=O)c3ccc(F)c(F)c3)c2c1 |r| Show InChI InChI=1S/C23H14F3N3O6S/c24-14-3-1-2-13(8-14)11-27-20-7-5-16(29(32)33)9-17(20)23(22(27)31)28(21(30)12-36(23,34)35)15-4-6-18(25)19(26)10-15/h1-10H,11-12H2/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341979

(5-(Biphenyl-4-carbonyl)-1,3,4,5-tetrahydro-thiopyr...)Show SMILES OS(=O)(=O)c1ccc2n(C(=O)c3ccc(cc3)-c3ccccc3)c3CCSCc3c2c1 Show InChI InChI=1S/C24H19NO4S2/c26-24(18-8-6-17(7-9-18)16-4-2-1-3-5-16)25-22-11-10-19(31(27,28)29)14-20(22)21-15-30-13-12-23(21)25/h1-11,14H,12-13,15H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341980

(3-Benzyl-1-(biphenyl-4-carbonyl)-2-methyl-1H-indol...)Show SMILES Cc1c(Cc2ccccc2)c2cc(ccc2n1C(=O)c1ccc(cc1)-c1ccccc1)S(O)(=O)=O Show InChI InChI=1S/C29H23NO4S/c1-20-26(18-21-8-4-2-5-9-21)27-19-25(35(32,33)34)16-17-28(27)30(20)29(31)24-14-12-23(13-15-24)22-10-6-3-7-11-22/h2-17,19H,18H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341981
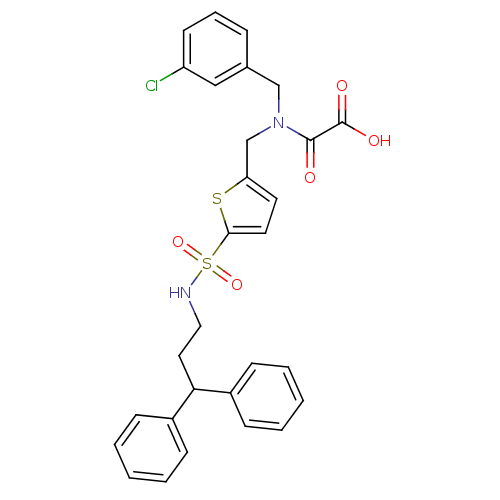
(2-((3-chlorobenzyl)((5-(N-(3,3-diphenylpropyl)sulf...)Show SMILES OC(=O)C(=O)N(Cc1ccc(s1)S(=O)(=O)NCCC(c1ccccc1)c1ccccc1)Cc1cccc(Cl)c1 Show InChI InChI=1S/C29H27ClN2O5S2/c30-24-13-7-8-21(18-24)19-32(28(33)29(34)35)20-25-14-15-27(38-25)39(36,37)31-17-16-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-15,18,26,31H,16-17,19-20H2,(H,34,35) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50342000
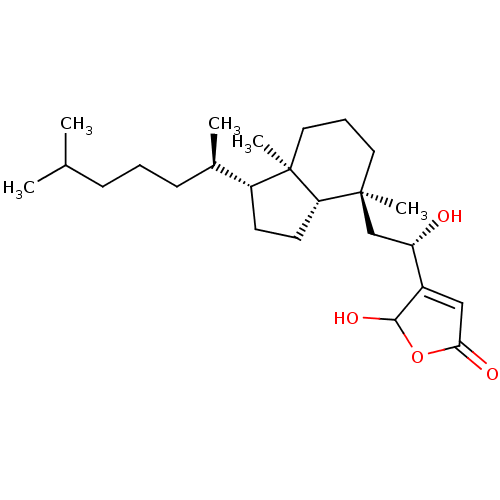
(4-((S)-2-((1R,3aS,4S,7aR)-4,7a-dimethyl-1-((R)-6-m...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@@H]2[C@]1(C)CCC[C@@]2(C)C[C@H](O)C1=CC(=O)OC1O |r,t:24| Show InChI InChI=1S/C25H42O4/c1-16(2)8-6-9-17(3)19-10-11-21-24(4,12-7-13-25(19,21)5)15-20(26)18-14-22(27)29-23(18)28/h14,16-17,19-21,23,26,28H,6-13,15H2,1-5H3/t17-,19-,20+,21+,23?,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25A |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341982

(5-(2-cyclohexyl-4-((1-(6-(4-fluoro-2-methylphenyls...)Show SMILES Cc1cc(F)ccc1S(=O)(=O)NCCCCCCn1cc(COc2ccc(-c3cc(no3)C(O)=O)c(c2)C2CCCCC2)nn1 Show InChI InChI=1S/C32H38FN5O6S/c1-22-17-24(33)11-14-31(22)45(41,42)34-15-7-2-3-8-16-38-20-25(35-37-38)21-43-26-12-13-27(30-19-29(32(39)40)36-44-30)28(18-26)23-9-5-4-6-10-23/h11-14,17-20,23,34H,2-10,15-16,21H2,1H3,(H,39,40) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50341999
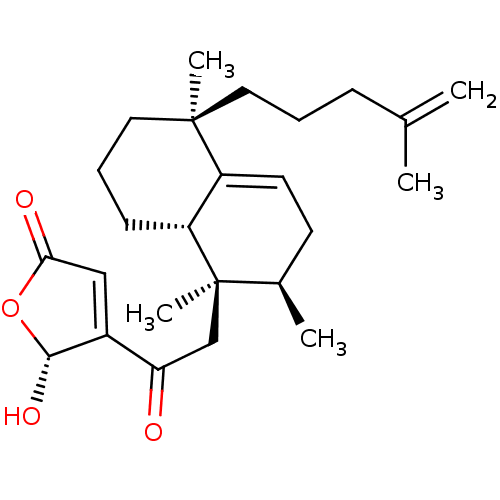
((R)-5-hydroxy-4-(2-((1R,2R,5S,8aS)-1,2,5-trimethyl...)Show SMILES C[C@@H]1CC=C2[C@@H](CCC[C@@]2(C)CCCC(C)=C)[C@]1(C)CC(=O)C1=CC(=O)O[C@H]1O |r,c:3,t:24| Show InChI InChI=1S/C25H36O4/c1-16(2)8-6-12-24(4)13-7-9-20-19(24)11-10-17(3)25(20,5)15-21(26)18-14-22(27)29-23(18)28/h11,14,17,20,23,28H,1,6-10,12-13,15H2,2-5H3/t17-,20-,23-,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25A |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50253622

(2-[4-(4-chlorophenyl)-5-p-tolyl-4H-[1,2,4]triazol-...)Show SMILES Cc1ccc(cc1)-c1nnc(SCC(=O)c2ccc(O)c(O)c2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H18ClN3O3S/c1-14-2-4-15(5-3-14)22-25-26-23(27(22)18-9-7-17(24)8-10-18)31-13-21(30)16-6-11-19(28)20(29)12-16/h2-12,28-29H,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25A |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM50184288
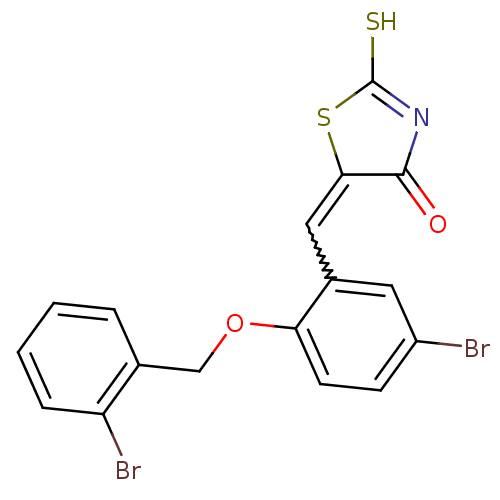
(5-(2-(2-bromobenzyloxy)-5-bromobenzylidene)-2-thio...)Show SMILES SC1=NC(=O)C(S1)=Cc1cc(Br)ccc1OCc1ccccc1Br |w:7.8,t:1| Show InChI InChI=1S/C17H11Br2NO2S2/c18-12-5-6-14(22-9-10-3-1-2-4-13(10)19)11(7-12)8-15-16(21)20-17(23)24-15/h1-8H,9H2,(H,20,21,23) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PRL-3 |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Low molecular weight protein-tyrosine phosphatase A
(Mycobacterium tuberculosis) | BDBM84499
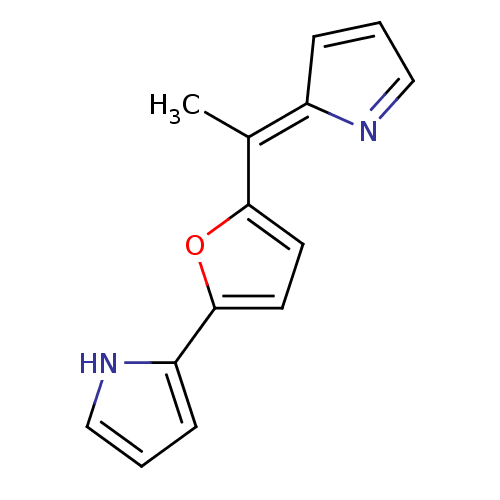
(2-(5-{1-[(2Z)-2H-pyrrol-2-ylidene]ethyl}furan-2-yl...)Show SMILES C\C(=C1/C=CC=N1)c1ccc(o1)-c1ccc[nH]1 |c:3,5| Show InChI InChI=1S/C14H12N2O/c1-10(11-4-2-8-15-11)13-6-7-14(17-13)12-5-3-9-16-12/h2-9,16H,1H3/b11-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpA |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50342001

(1-(biphenyl-4-yl)-3,4-bis(2-hydroxyethylthio)-1H-p...)Show SMILES OCCSC1=C(SCCO)C(=O)N(C1=O)c1ccc(cc1)-c1ccccc1 |c:4| Show InChI InChI=1S/C20H19NO4S2/c22-10-12-26-17-18(27-13-11-23)20(25)21(19(17)24)16-8-6-15(7-9-16)14-4-2-1-3-5-14/h1-9,22-23H,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25A |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341978
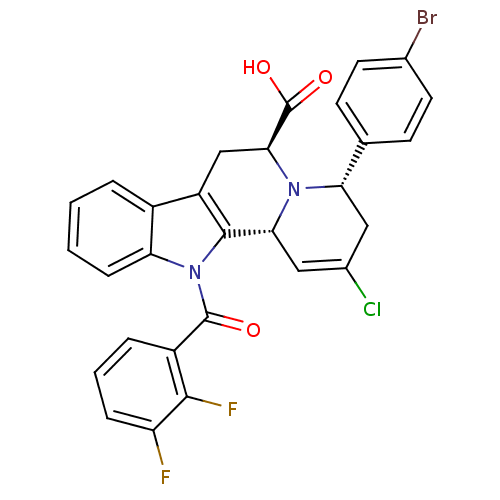
((4S,6S,12bR)-4-(4-bromophenyl)-2-chloro-12-(2,3-di...)Show SMILES OC(=O)[C@@H]1Cc2c([C@H]3C=C(Cl)C[C@H](N13)c1ccc(Br)cc1)n(C(=O)c1cccc(F)c1F)c1ccccc21 |r,t:8| Show InChI InChI=1S/C29H20BrClF2N2O3/c30-16-10-8-15(9-11-16)23-12-17(31)13-24-27-20(14-25(29(37)38)34(23)24)18-4-1-2-7-22(18)35(27)28(36)19-5-3-6-21(32)26(19)33/h1-11,13,23-25H,12,14H2,(H,37,38)/t23-,24+,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50341986
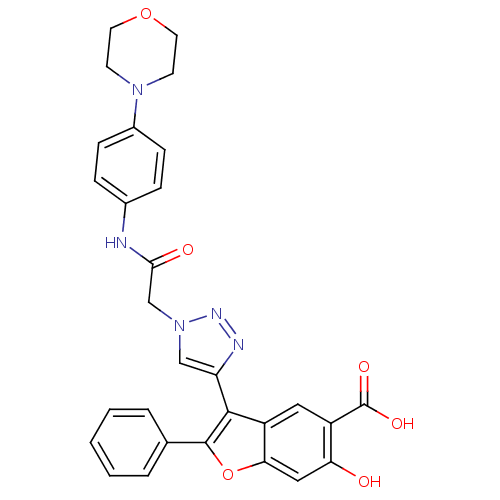
(6-hydroxy-3-(1-(2-(4-morpholinophenylamino)-2-oxoe...)Show SMILES OC(=O)c1cc2c(-c3cn(CC(=O)Nc4ccc(cc4)N4CCOCC4)nn3)c(oc2cc1O)-c1ccccc1 Show InChI InChI=1S/C29H25N5O6/c35-24-15-25-22(14-21(24)29(37)38)27(28(40-25)18-4-2-1-3-5-18)23-16-34(32-31-23)17-26(36)30-19-6-8-20(9-7-19)33-10-12-39-13-11-33/h1-9,14-16,35H,10-13,17H2,(H,30,36)(H,37,38) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis ptpB |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50333649
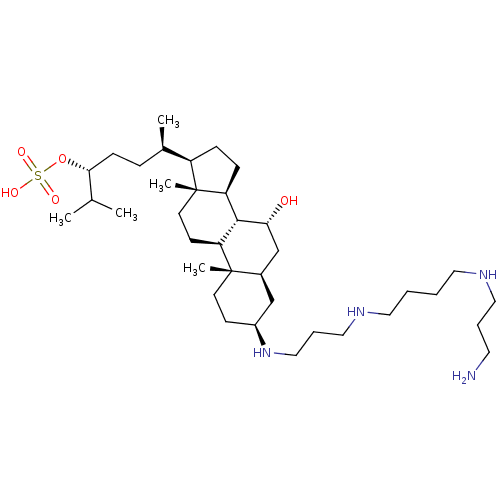
((3R,6R)-6-((3S,5R,7R,8R,9S,10S,13R,14S,17R)-3-(3-(...)Show SMILES CC(C)[C@@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)NCCCNCCCCNCCCN)OS(O)(=O)=O |r| Show InChI InChI=1S/C37H72N4O5S/c1-26(2)34(46-47(43,44)45)13-10-27(3)30-11-12-31-35-32(15-17-37(30,31)5)36(4)16-14-29(24-28(36)25-33(35)42)41-23-9-22-40-20-7-6-19-39-21-8-18-38/h26-35,39-42H,6-25,38H2,1-5H3,(H,43,44,45)/t27-,28-,29+,30-,31+,32+,33-,34-,35+,36+,37-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50209683
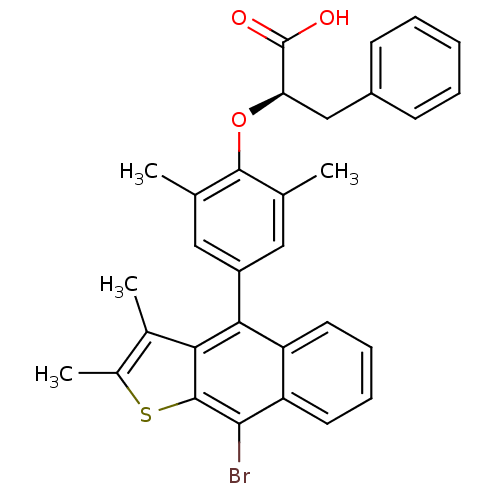
((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50342005

((5R)-2-((3S)-1-(((R)-3-hydroxy-5-oxo-4-palmitoyl-2...)Show SMILES CCCCCCCCCCCCCCCC(=O)C1C(=O)O[C@H](COC(=O)C(C)[C@H](CC)OCCOC(=O)C2C(=O)O[C@H](CO)C2=O)C1=O |r| Show InChI InChI=1S/C35H54O13/c1-4-6-7-8-9-10-11-12-13-14-15-16-17-18-24(37)28-31(39)27(48-34(28)42)22-46-32(40)23(3)25(5-2)44-19-20-45-33(41)29-30(38)26(21-36)47-35(29)43/h23,25-29,36H,4-22H2,1-3H3/t23?,25-,26+,27+,28?,29?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50342000

(4-((S)-2-((1R,3aS,4S,7aR)-4,7a-dimethyl-1-((R)-6-m...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@@H]2[C@]1(C)CCC[C@@]2(C)C[C@H](O)C1=CC(=O)OC1O |r,t:24| Show InChI InChI=1S/C25H42O4/c1-16(2)8-6-9-17(3)19-10-11-21-24(4,12-7-13-25(19,21)5)15-20(26)18-14-22(27)29-23(18)28/h14,16-17,19-21,23,26,28H,6-13,15H2,1-5H3/t17-,19-,20+,21+,23?,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50341996
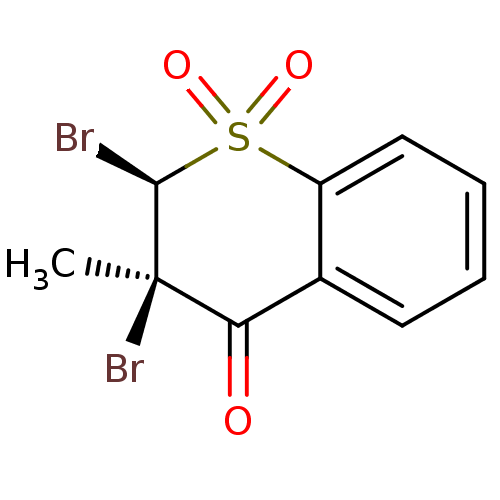
((2R,3R)-2,3-Dibromo-3-methyl-1,1-dioxo-1lambda*6*-...)Show SMILES C[C@]1(Br)[C@@H](Br)S(=O)(=O)c2ccccc2C1=O |r| Show InChI InChI=1S/C10H8Br2O3S/c1-10(12)8(13)6-4-2-3-5-7(6)16(14,15)9(10)11/h2-5,9H,1H3/t9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25A |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50253622

(2-[4-(4-chlorophenyl)-5-p-tolyl-4H-[1,2,4]triazol-...)Show SMILES Cc1ccc(cc1)-c1nnc(SCC(=O)c2ccc(O)c(O)c2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H18ClN3O3S/c1-14-2-4-15(5-3-14)22-25-26-23(27(22)18-9-7-17(24)8-10-18)31-13-21(30)16-6-11-19(28)20(29)12-16/h2-12,28-29H,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50342002

((2S,3aR,6S,6aR)-N-(cyclohexylmethyl)-6-(5-((R)-3-(...)Show SMILES C(N[C@@H]1C[C@H]2OC[C@@H]([C@H]2O1)n1nnnc1-c1cccc(CN2CCCC2)c1)C1CCCCC1 |r| Show InChI InChI=1S/C25H36N6O2/c1-2-7-18(8-3-1)15-26-23-14-22-24(33-23)21(17-32-22)31-25(27-28-29-31)20-10-6-9-19(13-20)16-30-11-4-5-12-30/h6,9-10,13,18,21-24,26H,1-5,7-8,11-12,14-17H2/t21-,22+,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of SHP-2 |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50166435
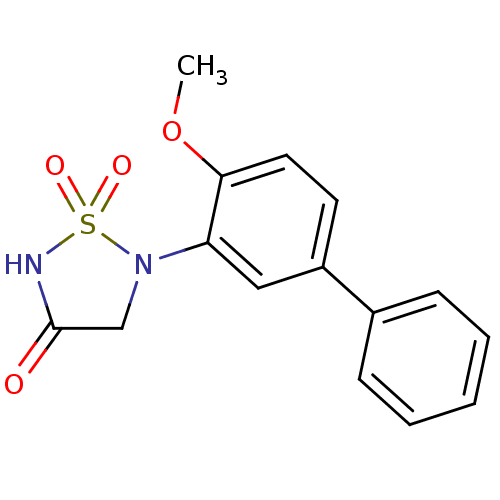
(5-(4-METHOXYBIPHENYL-3-YL)-1,2,5-THIADIAZOLIDIN-3-...)Show InChI InChI=1S/C15H14N2O4S/c1-21-14-8-7-12(11-5-3-2-4-6-11)9-13(14)17-10-15(18)16-22(17,19)20/h2-9H,10H2,1H3,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM84499

(2-(5-{1-[(2Z)-2H-pyrrol-2-ylidene]ethyl}furan-2-yl...)Show SMILES C\C(=C1/C=CC=N1)c1ccc(o1)-c1ccc[nH]1 |c:3,5| Show InChI InChI=1S/C14H12N2O/c1-10(11-4-2-8-15-11)13-6-7-14(17-13)12-5-3-9-16-12/h2-9,16H,1H3/b11-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50197854

(3-(4-heptadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H40O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h2-19H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data