

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342765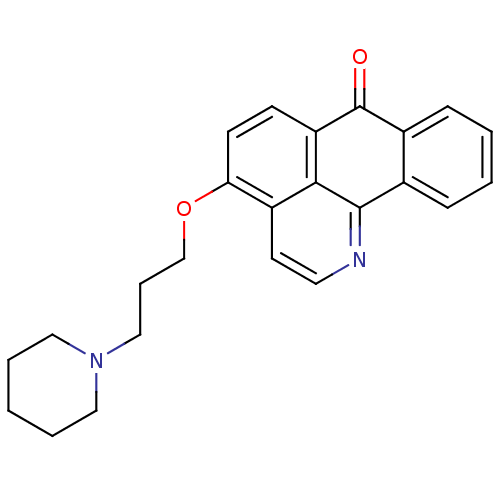 (4-(3-(Piperidin-1-yl)propoxy)-7H-dibenzo[de,h]quin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342763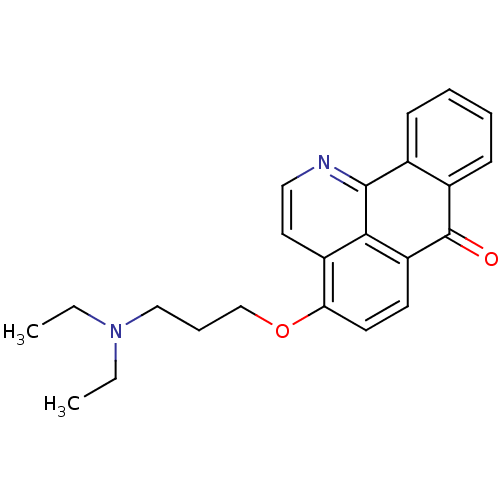 (4-(3-(Diethylamino)propoxy)-7H-dibenzo[de,h]quinol...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342764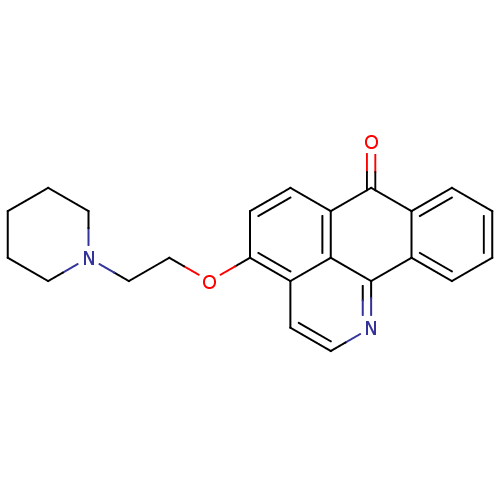 (4-(2-(Piperidin-1-yl)ethoxy)-7H-dibenzo[de,h]quino...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342761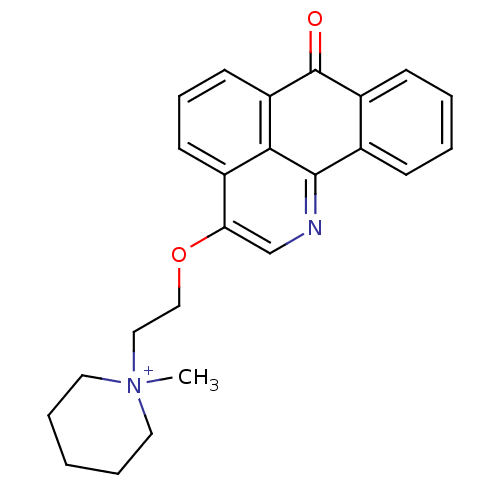 (1-methyl-1-(2-(7-oxo-7H-dibenzo[de,h]quinolin-3-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342760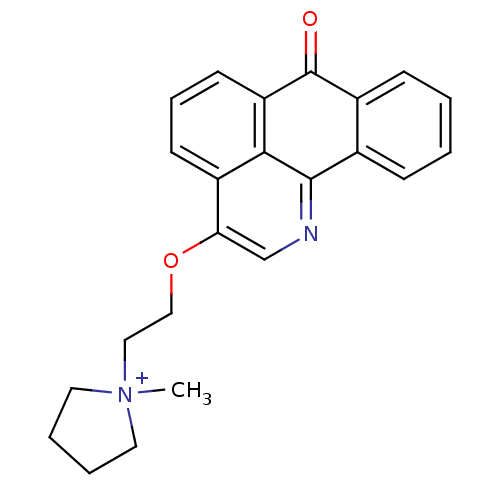 (1-methyl-1-(2-(7-oxo-7H-dibenzo[de,h]quinolin-3-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342762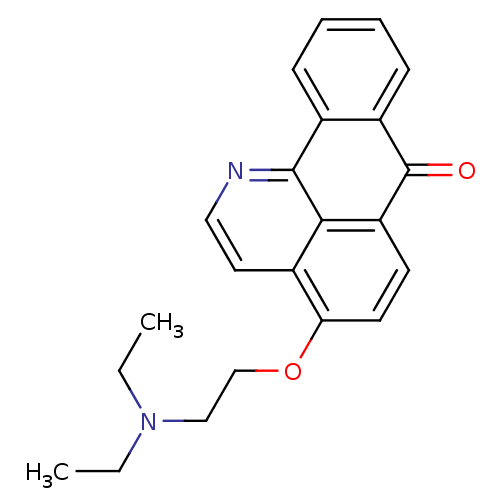 (4-(2-(Diethylamino)ethoxy)-7H-dibenzo[de,h]quinoli...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342761 (1-methyl-1-(2-(7-oxo-7H-dibenzo[de,h]quinolin-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342759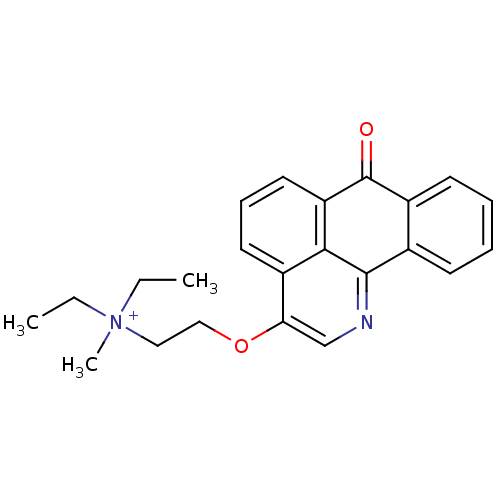 (CHEMBL1770197 | N,N-diethyl-N-methyl-2-(7-oxo-7H-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342760 (1-methyl-1-(2-(7-oxo-7H-dibenzo[de,h]quinolin-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342757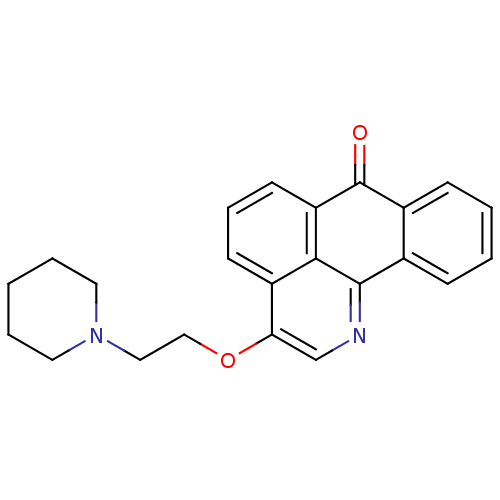 (3-(2-(Piperidin-1-yl)ethoxy)-7H-dibenzo[de,h]quino...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342768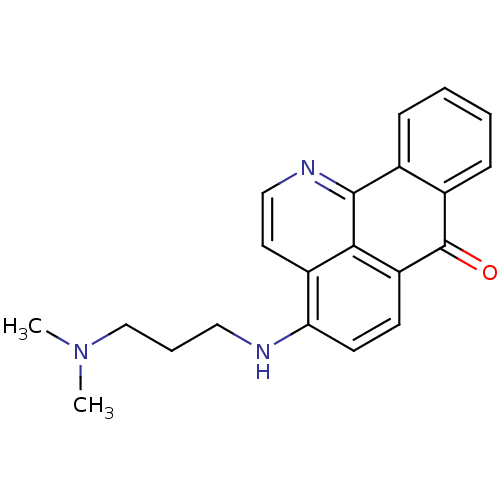 (4-(3-(Dimethylamino)propylamino)-7H-dibenzo[de,h]q...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342767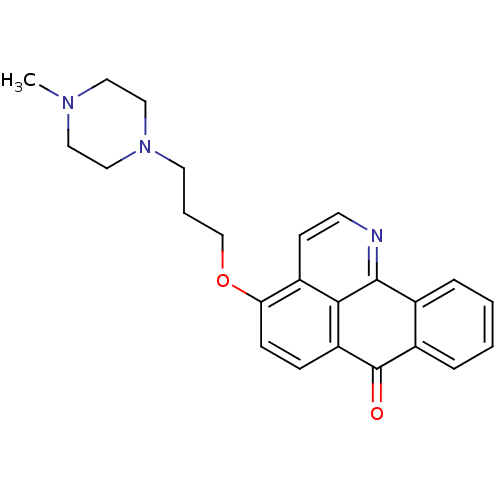 (4-(3-(4-Methylpiperazin-1-yl)propoxy)-7H-dibenzo[d...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 405 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342766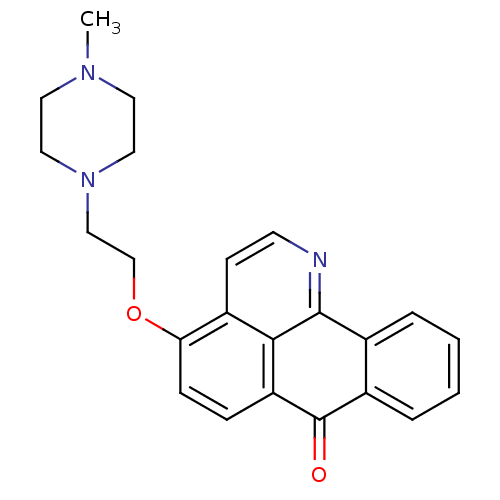 (4-(2-(4-Methylpiperazin-1-yl)ethoxy)-7H-dibenzo[de...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342756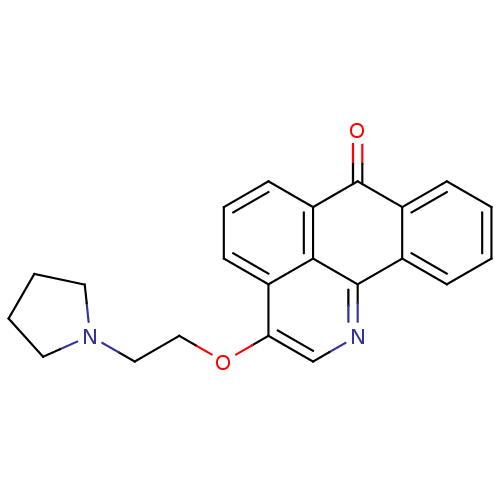 (3-(2-(Pyrrolidin-1-yl)ethoxy)-7H-dibenzo[de,h]quin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 471 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342755 (3-(2-(Diethylamino)ethoxy)-7H-dibenzo[de,h]quinoli...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342759 (CHEMBL1770197 | N,N-diethyl-N-methyl-2-(7-oxo-7H-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342757 (3-(2-(Piperidin-1-yl)ethoxy)-7H-dibenzo[de,h]quino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342756 (3-(2-(Pyrrolidin-1-yl)ethoxy)-7H-dibenzo[de,h]quin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342765 (4-(3-(Piperidin-1-yl)propoxy)-7H-dibenzo[de,h]quin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 905 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342758 (CHEMBL1770198 | N,N,N-trimethyl-2-(7-oxo-7H-dibenz...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 933 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342758 (CHEMBL1770198 | N,N,N-trimethyl-2-(7-oxo-7H-dibenz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 972 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342755 (3-(2-(Diethylamino)ethoxy)-7H-dibenzo[de,h]quinoli...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342754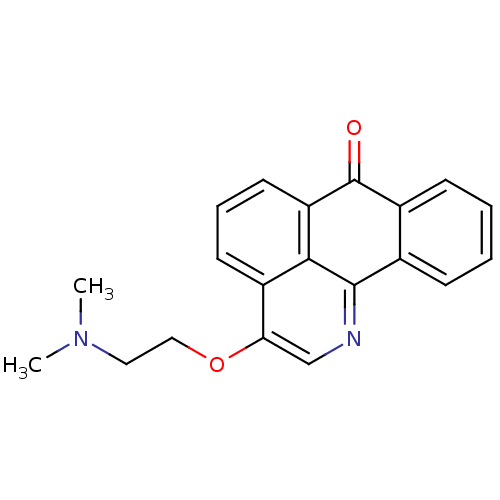 (3-(2-(Dimethylamino)ethoxy)-7H-dibenzo[de,h]quinol...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342754 (3-(2-(Dimethylamino)ethoxy)-7H-dibenzo[de,h]quinol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342764 (4-(2-(Piperidin-1-yl)ethoxy)-7H-dibenzo[de,h]quino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342763 (4-(3-(Diethylamino)propoxy)-7H-dibenzo[de,h]quinol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342766 (4-(2-(4-Methylpiperazin-1-yl)ethoxy)-7H-dibenzo[de...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342762 (4-(2-(Diethylamino)ethoxy)-7H-dibenzo[de,h]quinoli...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342768 (4-(3-(Dimethylamino)propylamino)-7H-dibenzo[de,h]q...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50342767 (4-(3-(4-Methylpiperazin-1-yl)propoxy)-7H-dibenzo[d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||