| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50342759 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_744406 (CHEMBL1772360) |
|---|
| IC50 | 708±n/a nM |
|---|
| Citation |  Li, YP; Ning, FX; Yang, MB; Li, YC; Nie, MH; Ou, TM; Tan, JH; Huang, SL; Li, D; Gu, LQ; Huang, ZS Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur J Med Chem46:1572-81 (2011) [PubMed] Article Li, YP; Ning, FX; Yang, MB; Li, YC; Nie, MH; Ou, TM; Tan, JH; Huang, SL; Li, D; Gu, LQ; Huang, ZS Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur J Med Chem46:1572-81 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | BCHE | Butyrylcholinesterase (BuChE) | CHLE_HORSE | Cholinesterase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 65643.35 |
|---|
| Organism: | Equus caballus (Horse) |
|---|
| Description: | P81908 |
|---|
| Residue: | 574 |
|---|
| Sequence: | EEDIIITTKNGKVRGMNLPVLGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSNIWNATK
YANSCYQNTDQSFPGFLGSEMWNPNTELSEDCLYLNVWIPAPKPKNATVMIWIYGGGFQT
GTSSLPVYDGKFLARVERVIVVSMNYRVGALGFLALSENPEAPGNMGLFDQQLALQWVQK
NIAAFGGNPRSVTLFGESAGAASVSLHLLSPRSQPLFTRAILQSGSSNAPWAVTSLYEAR
NRTLTLAKRMGCSRDNETEMIKCLRDKDPQEILLNEVFVVPYDTLLSVNFGPTVDGDFLT
DMPDTLLQLGQFKRTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPR
VSEFGRESILFHYMDWLDDQRAENYREALDDVVGDYNIICPALEFTRKFSELGNDAFFYY
FEHRSTKLPWPEWMGVMHGYEIEFVFGLPLERRVNYTRAEEILSRSIMKRWANFAKYGNP
NGTQNNSTRWPVFKSTEQKYLTLNTESPKVYTKLRAQQCRFWTLFFPKVLELTGNIDEAE
REWKAGFHRWNNYMMDWKNQFNDYTSKKESCSDF
|
|
|
|---|
| BDBM50342759 |
|---|
| n/a |
|---|
| Name | BDBM50342759 |
|---|
| Synonyms: | CHEMBL1770197 | N,N-diethyl-N-methyl-2-(7-oxo-7H-dibenzo[de,h]quinolin-3-yloxy)ethanaminium iodide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H25N2O2 |
|---|
| Mol. Mass. | 361.4563 |
|---|
| SMILES | CC[N+](C)(CC)CCOc1cnc2-c3ccccc3C(=O)c3cccc1c23 |
|---|
| Structure | 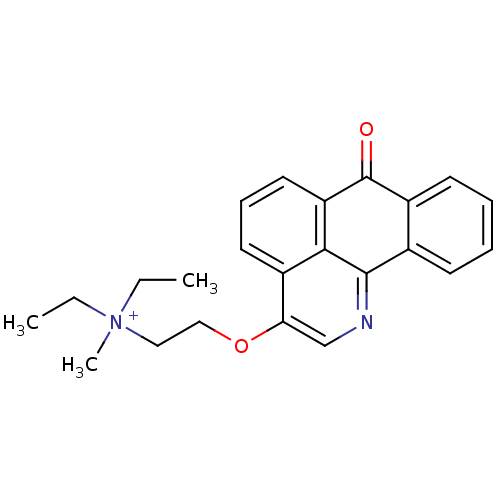
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Li, YP; Ning, FX; Yang, MB; Li, YC; Nie, MH; Ou, TM; Tan, JH; Huang, SL; Li, D; Gu, LQ; Huang, ZS Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur J Med Chem46:1572-81 (2011) [PubMed] Article
Li, YP; Ning, FX; Yang, MB; Li, YC; Nie, MH; Ou, TM; Tan, JH; Huang, SL; Li, D; Gu, LQ; Huang, ZS Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur J Med Chem46:1572-81 (2011) [PubMed] Article