

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293451 (1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293452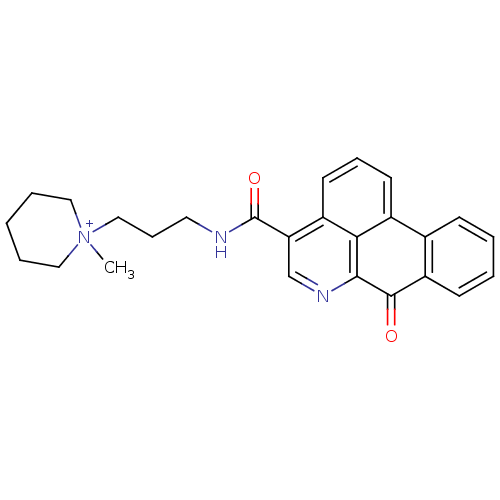 (1-methyl-1-(3-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211247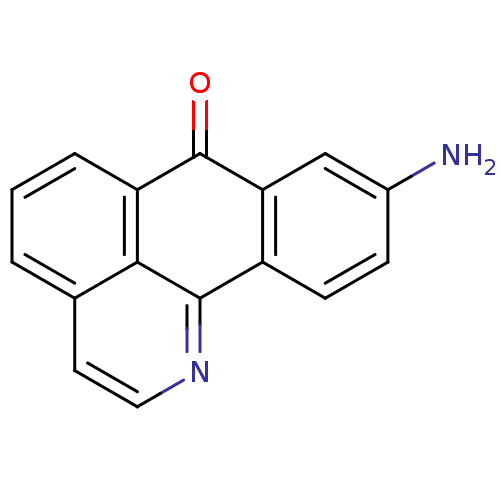 (9-Amino-1-azabenzanthrone | 9-amino-1-aza-benzo[de...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293443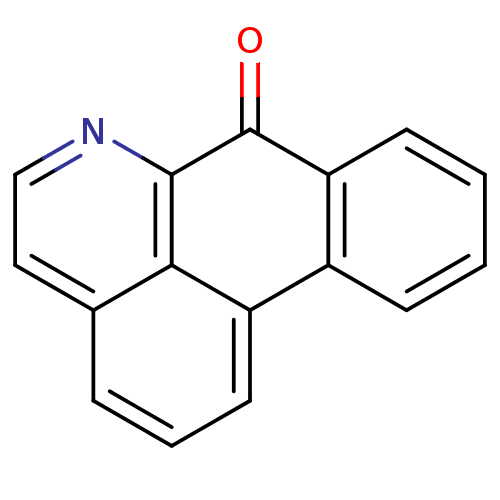 (7-Oxo-7H-dibenzo[de,g]quinoline | CHEMBL559502) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by LB plot | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293451 (1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433027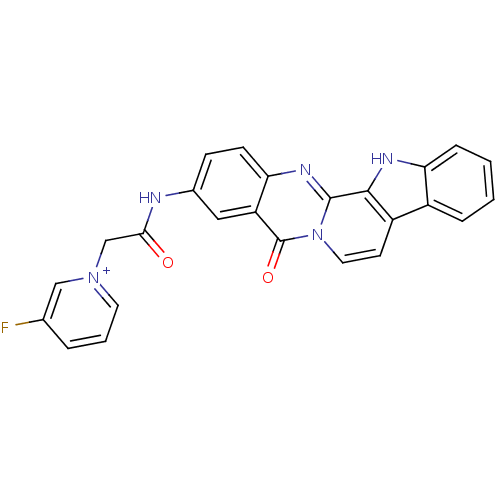 (CHEMBL2375941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433028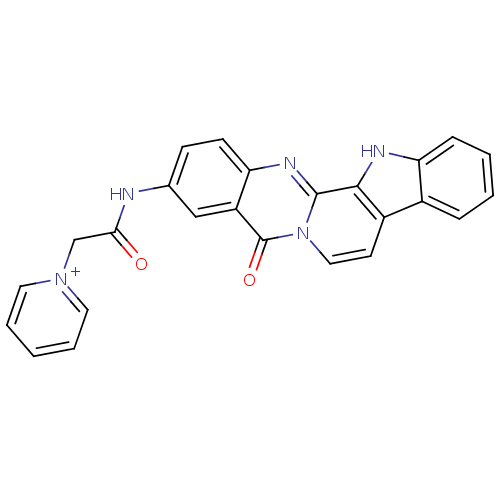 (CHEMBL2375940) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211239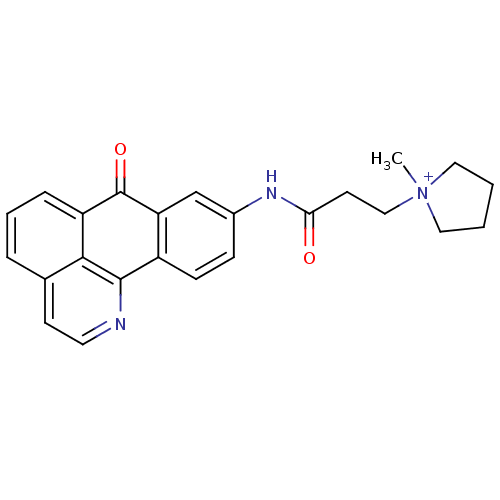 (1-methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 17: 3765-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.015 BindingDB Entry DOI: 10.7270/Q28C9X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211252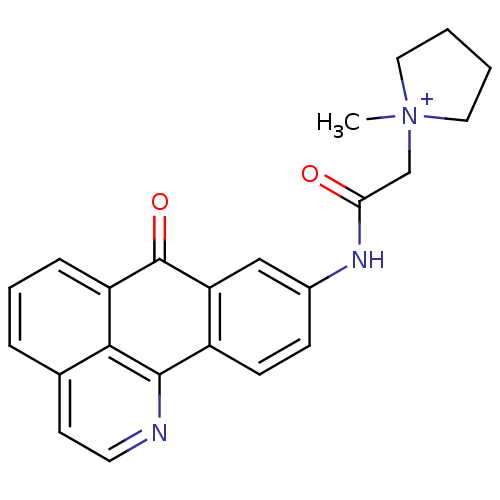 (1-methyl-1-[(7-oxo-7H-1-aza-benzo[de]anthracen-9-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 17: 3765-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.015 BindingDB Entry DOI: 10.7270/Q28C9X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433018 (CHEMBL2375923) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433019 (CHEMBL2375922) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211241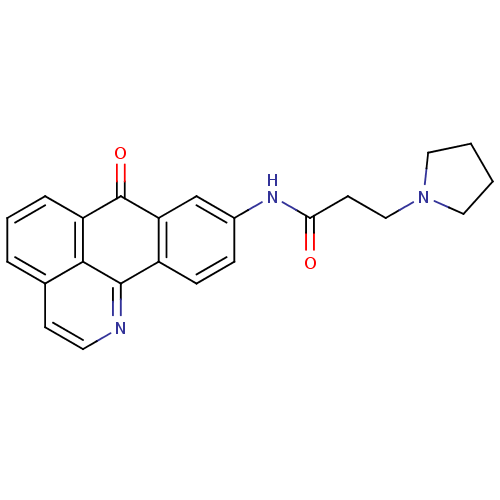 (9-[3-Pyrrolidinopropionamido]-1-azabenzanthrone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 17: 3765-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.015 BindingDB Entry DOI: 10.7270/Q28C9X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211250 (1-methyl-1-[3-(7-oxo-7H-1-aza-benzo[de]anthracen-9...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 17: 3765-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.015 BindingDB Entry DOI: 10.7270/Q28C9X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333767 (CHEMBL1644288 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50293446 (CHEMBL556249 | Diethyl-methyl-[2-(7-oxo-7H-1-aza-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50371216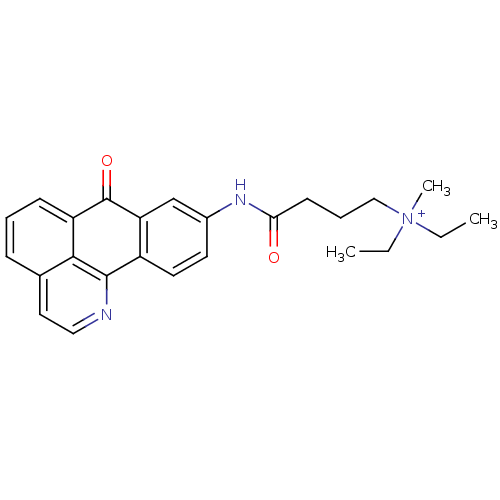 (CHEMBL554252) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 17: 3765-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.015 BindingDB Entry DOI: 10.7270/Q28C9X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333763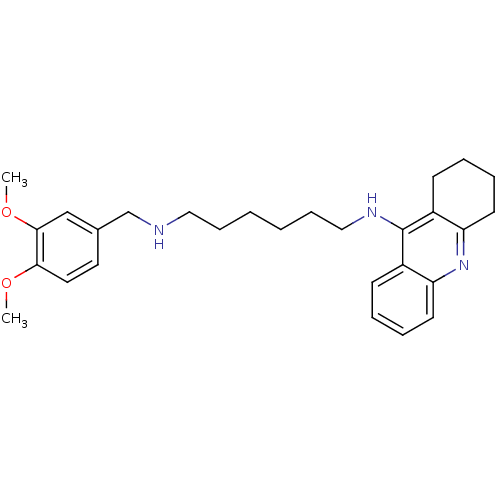 (CHEMBL1644292 | N1-(3,4-Dimethoxybenzyl)-N6-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433022 (CHEMBL2375919) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333765 (CHEMBL1644290 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353684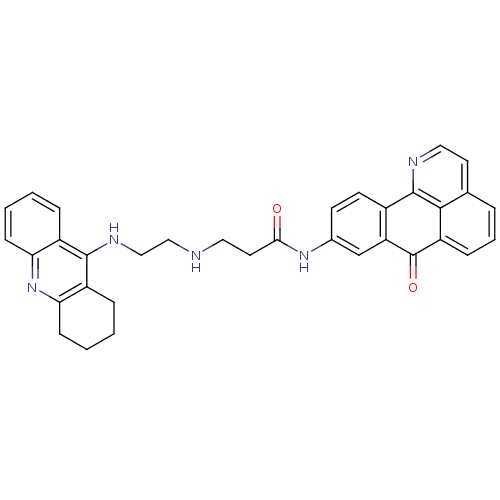 (CHEMBL1830627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345200 (2-Methoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-ylami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345205 (CHEMBL1783241 | N1-(Benzo[d][1,3]dioxol-5-ylmethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333766 (CHEMBL1644289 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433005 (CHEMBL2375936) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345201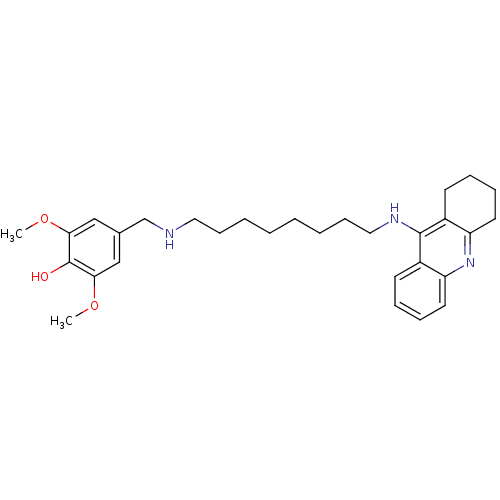 (2,6-Dimethoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50345200 (2-Methoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-ylami...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50345201 (2,6-Dimethoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345206 (CHEMBL1783242 | N1-(Pyridin-4-ylmethyl)-N8-(1,2,3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50345206 (CHEMBL1783242 | N1-(Pyridin-4-ylmethyl)-N8-(1,2,3,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333764 (CHEMBL1644291 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211253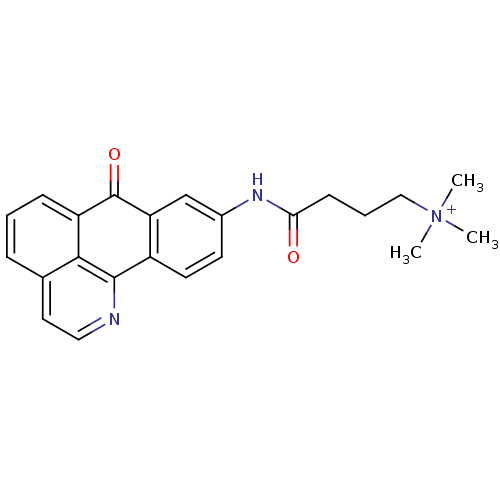 (CHEMBL245668 | trimethyl-[3-(7-oxo-7H-1-aza-benzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 17: 3765-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.015 BindingDB Entry DOI: 10.7270/Q28C9X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211244 (CHEMBL395926 | Trimethyl-[2-(7-oxo-7H-1-aza-benzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 44: 2523-32 (2009) Article DOI: 10.1016/j.ejmech.2009.01.021 BindingDB Entry DOI: 10.7270/Q2Z89CFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211244 (CHEMBL395926 | Trimethyl-[2-(7-oxo-7H-1-aza-benzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 17: 3765-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.015 BindingDB Entry DOI: 10.7270/Q28C9X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345204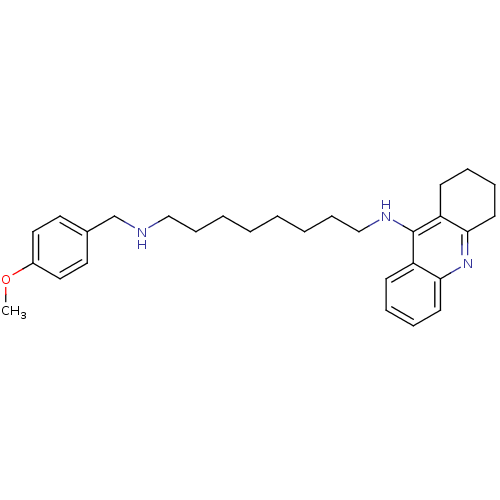 (CHEMBL1783240 | N1-(4-Methoxybenzyl)-N8-(1,2,3,4-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345202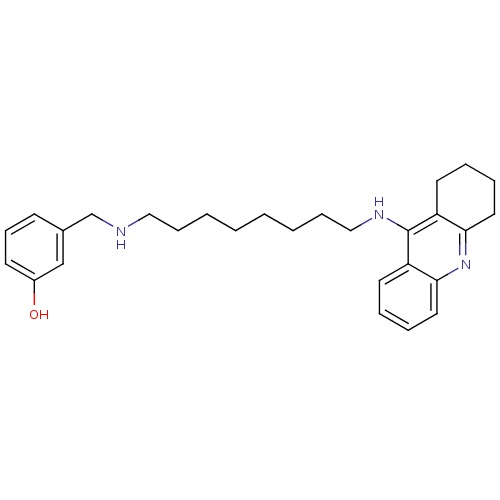 (3-((8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333771 (CHEMBL1644284 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345199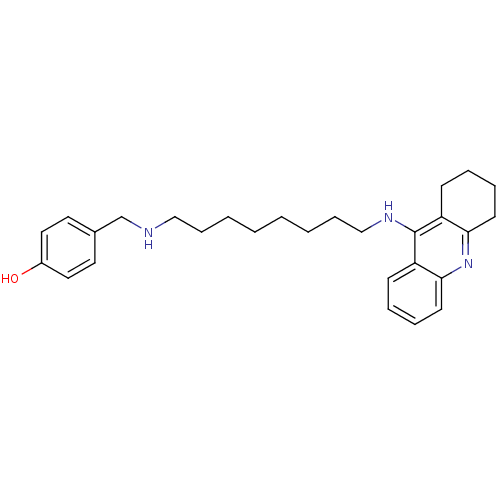 (4-((8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333762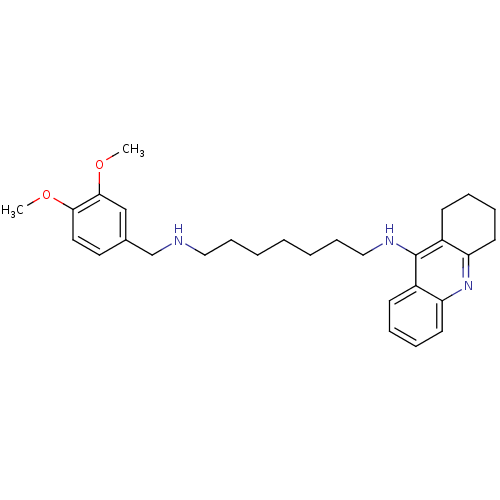 (CHEMBL1644293 | N1-(3,4-Dimethoxybenzyl)-N7-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333761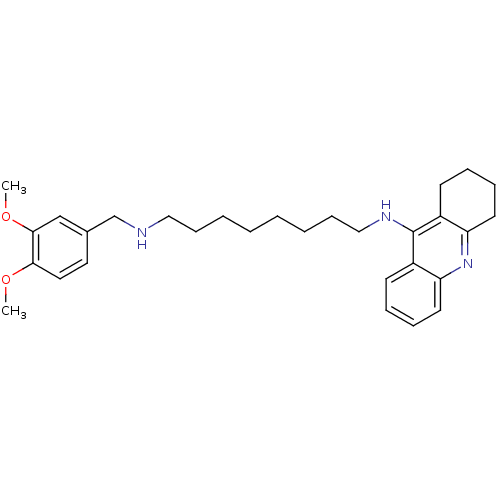 (CHEMBL1644294 | N1-(3,4-Dimethoxybenzyl)-N8-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333760 (CHEMBL1644295 | N1-(3,4-Dimethoxybenzyl)-N9-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50345205 (CHEMBL1783241 | N1-(Benzo[d][1,3]dioxol-5-ylmethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211254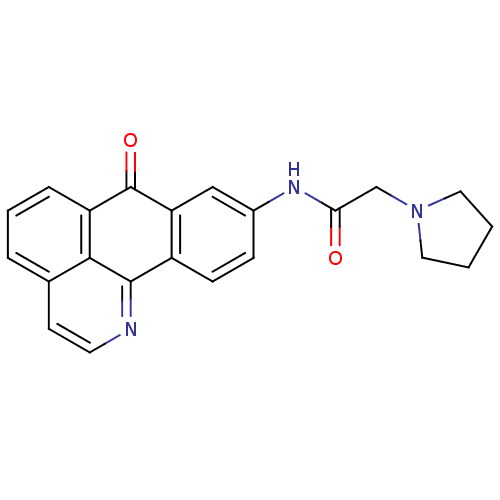 (9-(Pyrrolidinoacetamido)-1-azabenzanthrone | CHEMB...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 17: 3765-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.015 BindingDB Entry DOI: 10.7270/Q28C9X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50345199 (4-((8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345197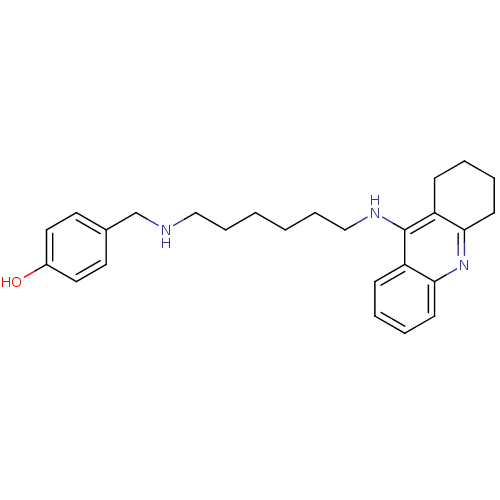 (4-((6-(1,2,3,4-Tetrahydroacridin-9-ylamino)hexylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333769 (CHEMBL1644286 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333769 (CHEMBL1644286 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433026 (CHEMBL2375942) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345198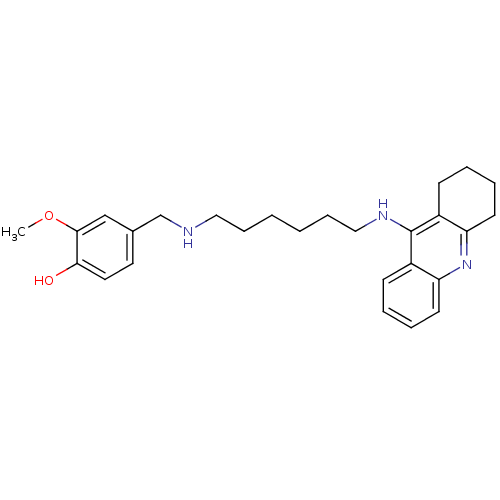 (2-Methoxy-4-((6-(1,2,3,4-tetrahydroacridin-9-ylami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433017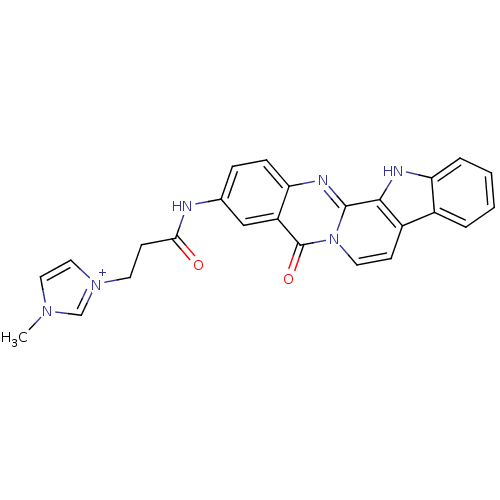 (CHEMBL2375924) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333770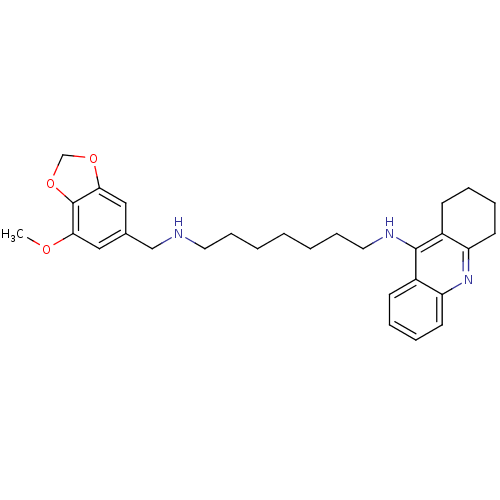 (CHEMBL1644285 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 532 total ) | Next | Last >> |