

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433027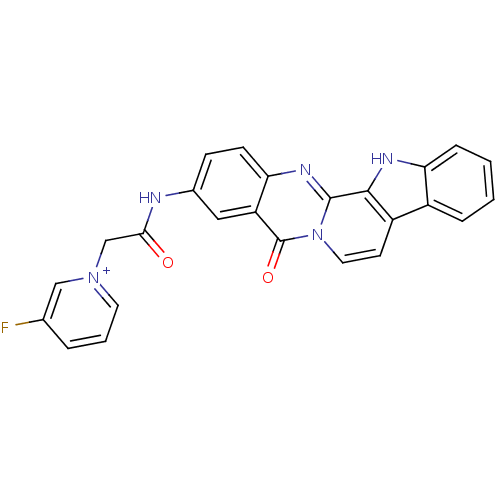 (CHEMBL2375941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433028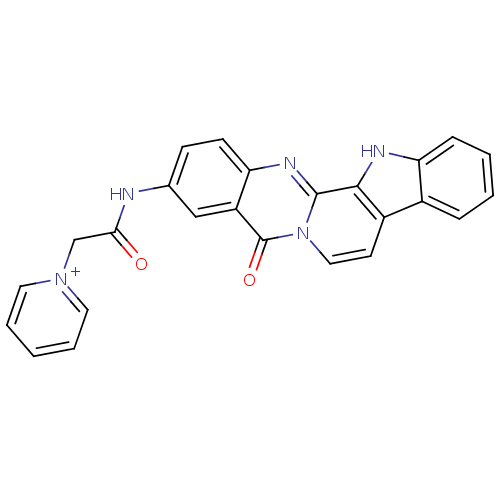 (CHEMBL2375940) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433018 (CHEMBL2375923) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433019 (CHEMBL2375922) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333767 (CHEMBL1644288 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333763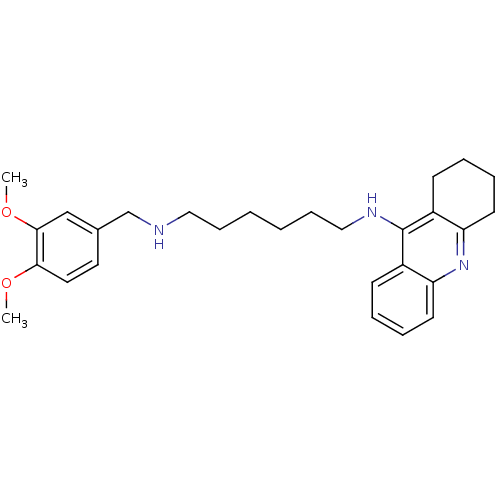 (CHEMBL1644292 | N1-(3,4-Dimethoxybenzyl)-N6-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433022 (CHEMBL2375919) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333765 (CHEMBL1644290 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333766 (CHEMBL1644289 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433005 (CHEMBL2375936) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333764 (CHEMBL1644291 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333771 (CHEMBL1644284 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333762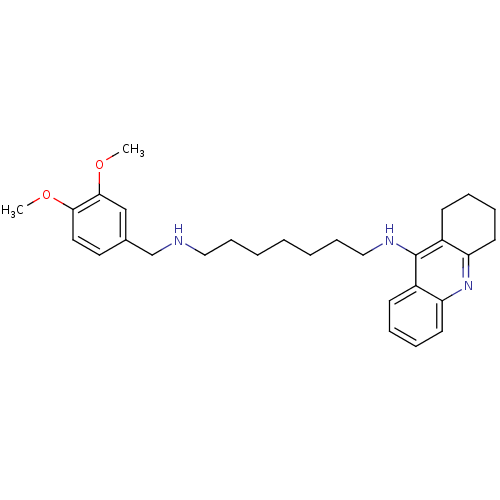 (CHEMBL1644293 | N1-(3,4-Dimethoxybenzyl)-N7-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333761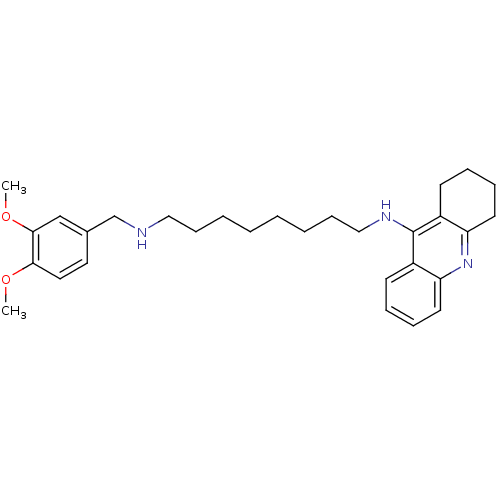 (CHEMBL1644294 | N1-(3,4-Dimethoxybenzyl)-N8-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333760 (CHEMBL1644295 | N1-(3,4-Dimethoxybenzyl)-N9-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333769 (CHEMBL1644286 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333769 (CHEMBL1644286 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433026 (CHEMBL2375942) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433017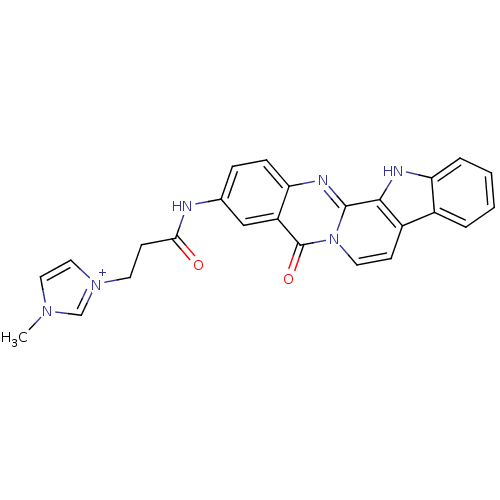 (CHEMBL2375924) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333770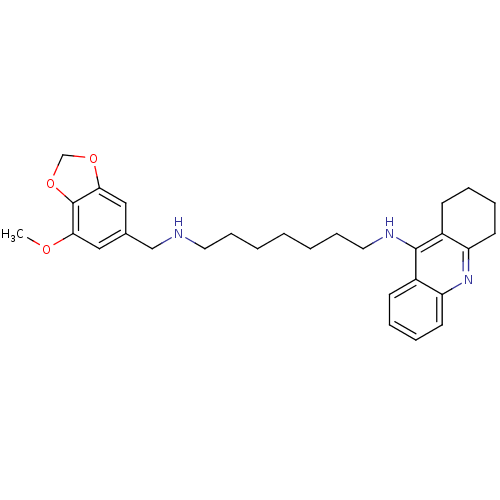 (CHEMBL1644285 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333765 (CHEMBL1644290 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuchE | Bioorg Med Chem Lett 18: 3790-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.039 BindingDB Entry DOI: 10.7270/Q2154GTQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316369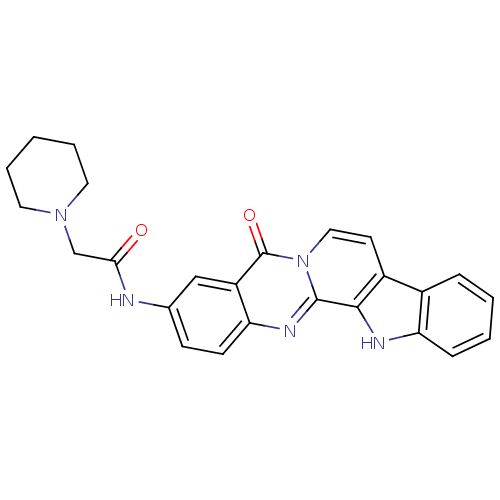 (3-(2-N-Piperidyl-acetamino)-7,8-dehydrorutaecarpin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description inhibition of electic eel AChE by Ellman's method | Eur J Med Chem 45: 1415-23 (2010) Article DOI: 10.1016/j.ejmech.2009.12.044 BindingDB Entry DOI: 10.7270/Q2CN742S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333761 (CHEMBL1644294 | N1-(3,4-Dimethoxybenzyl)-N8-(1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333768 (CHEMBL1644287 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433004 (CHEMBL2375937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylthiocholine chloride as substrate preincubated for 15 mins before substrate addition by... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433014 (CHEMBL2375927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433010 (CHEMBL2375931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333766 (CHEMBL1644289 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333770 (CHEMBL1644285 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333768 (CHEMBL1644287 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butylthiocholine chloride as substrate pretreated for 15 mins followed by substrate addition measured for 2 min... | Eur J Med Chem 130: 139-153 (2017) Article DOI: 10.1016/j.ejmech.2017.02.042 BindingDB Entry DOI: 10.7270/Q2P55R0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01058 BindingDB Entry DOI: 10.7270/Q2ZC86W8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333764 (CHEMBL1644291 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333771 (CHEMBL1644284 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM240807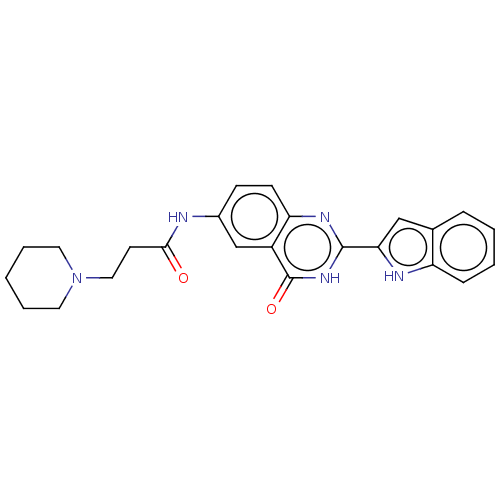 (N-(2-(1H-indol-2-yl)-4-oxo-3,4-dihydroquinazolin-6...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21.0 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Sun Yat-sen University | Assay Description All the assays were under 0.1 M KH2PO4/K2HPO4 buffer, pH 8.0, using a Shimadzu 2450 Spectrophotometer. Enzyme solutions were prepared to give 2 units... | J Enzyme Inhib Med Chem 28: 583-92 (2013) Article DOI: 10.3109/14756366.2012.663363 BindingDB Entry DOI: 10.7270/Q28914SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316366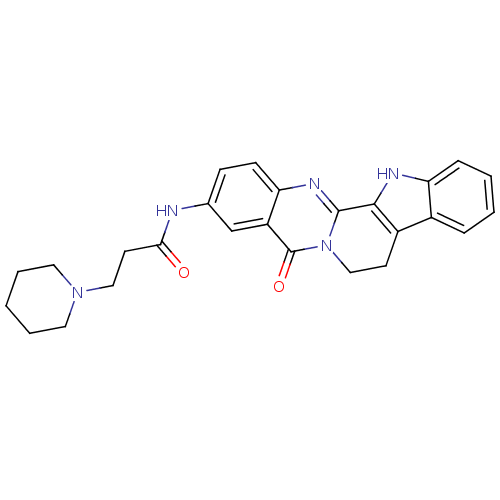 (3-(2-N-Piperidyl-propionamino)-rutaecarpine | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description inhibition of electic eel AChE by Ellman's method | Eur J Med Chem 45: 1415-23 (2010) Article DOI: 10.1016/j.ejmech.2009.12.044 BindingDB Entry DOI: 10.7270/Q2CN742S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50342765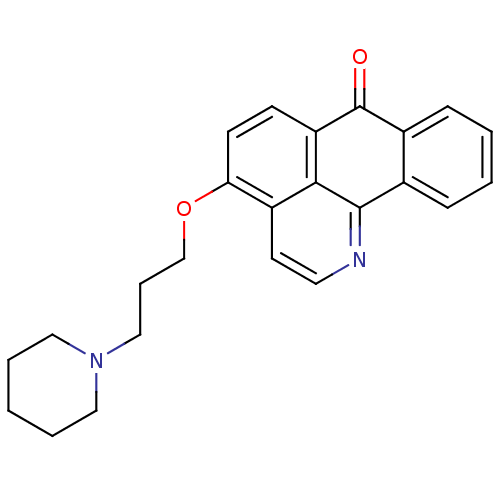 (4-(3-(Piperidin-1-yl)propoxy)-7H-dibenzo[de,h]quin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 46: 1572-81 (2011) Article DOI: 10.1016/j.ejmech.2011.02.005 BindingDB Entry DOI: 10.7270/Q2TM7BFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333767 (CHEMBL1644288 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380184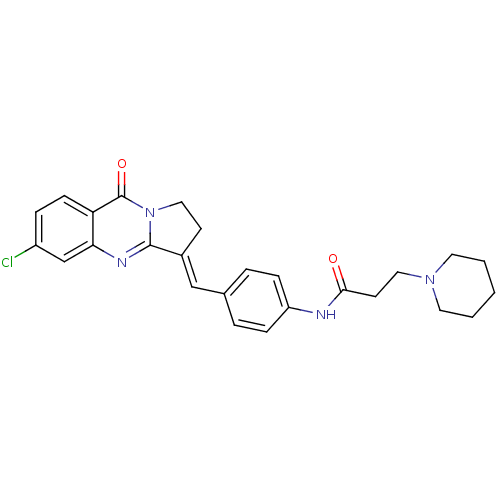 (CHEMBL2011200) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylcholine chloride as substrate preincubated for 15 mins before substrate addition by Ellma... | Bioorg Med Chem 20: 2527-34 (2012) Article DOI: 10.1016/j.bmc.2012.02.061 BindingDB Entry DOI: 10.7270/Q26111B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316368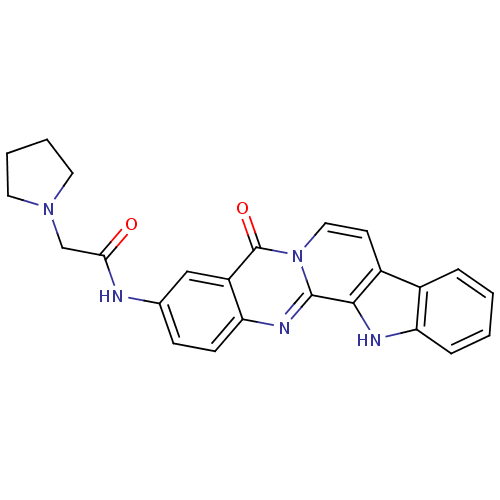 (3-(2-N-Pyrrolyl-acetamino)-7,8-dehydrorutaecarpine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description inhibition of electic eel AChE by Ellman's method | Eur J Med Chem 45: 1415-23 (2010) Article DOI: 10.1016/j.ejmech.2009.12.044 BindingDB Entry DOI: 10.7270/Q2CN742S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333762 (CHEMBL1644293 | N1-(3,4-Dimethoxybenzyl)-N7-(1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333760 (CHEMBL1644295 | N1-(3,4-Dimethoxybenzyl)-N9-(1,2,3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316365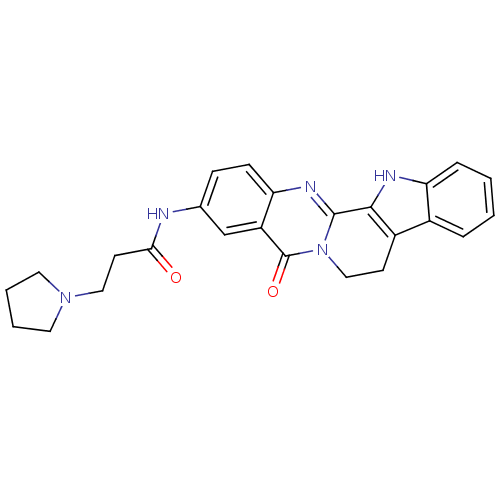 (3-(2-N-Pyrrolyl-propionamino)-rutaecarpine | CHEMB...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description inhibition of electic eel AChE by Ellman's method | Eur J Med Chem 45: 1415-23 (2010) Article DOI: 10.1016/j.ejmech.2009.12.044 BindingDB Entry DOI: 10.7270/Q2CN742S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333772 (CHEMBL1644283 | N1-((7-Methoxybenzo[d][1,3]dioxol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 1415-23 (2010) Article DOI: 10.1016/j.ejmech.2009.12.044 BindingDB Entry DOI: 10.7270/Q2CN742S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30.0 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Sun Yat-sen University | Assay Description All the assays were under 0.1 M KH2PO4/K2HPO4 buffer, pH 8.0, using a Shimadzu 2450 Spectrophotometer. Enzyme solutions were prepared to give 2 units... | J Enzyme Inhib Med Chem 28: 583-92 (2013) Article DOI: 10.3109/14756366.2012.663363 BindingDB Entry DOI: 10.7270/Q28914SJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 552 total ) | Next | Last >> |