 Found 105 hits of ic50 for UniProtKB: P12821
Found 105 hits of ic50 for UniProtKB: P12821 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme in Hog plasma |
J Med Chem 33: 1606-15 (1990)
BindingDB Entry DOI: 10.7270/Q2CF9QPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50017129

((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition against angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 4: 2673-2676 (1994)
Article DOI: 10.1016/S0960-894X(01)80694-6
BindingDB Entry DOI: 10.7270/Q2X34XXT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027132
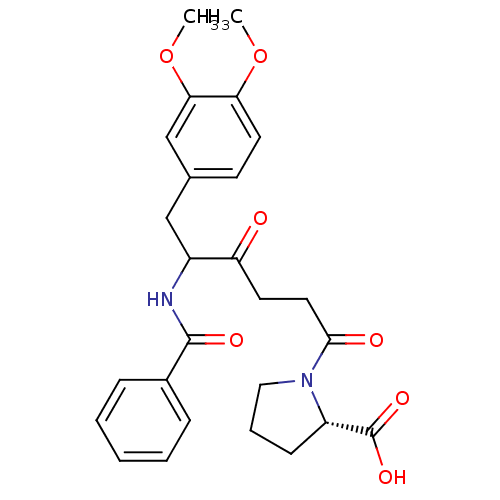
(1-[5-Benzoylamino-6-(3,4-dimethoxy-phenyl)-4-oxo-h...)Show SMILES COc1ccc(CC(NC(=O)c2ccccc2)C(=O)CCC(=O)N2CCC[C@H]2C(O)=O)cc1OC Show InChI InChI=1S/C26H30N2O7/c1-34-22-12-10-17(16-23(22)35-2)15-19(27-25(31)18-7-4-3-5-8-18)21(29)11-13-24(30)28-14-6-9-20(28)26(32)33/h3-5,7-8,10,12,16,19-20H,6,9,11,13-15H2,1-2H3,(H,27,31)(H,32,33)/t19?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027142
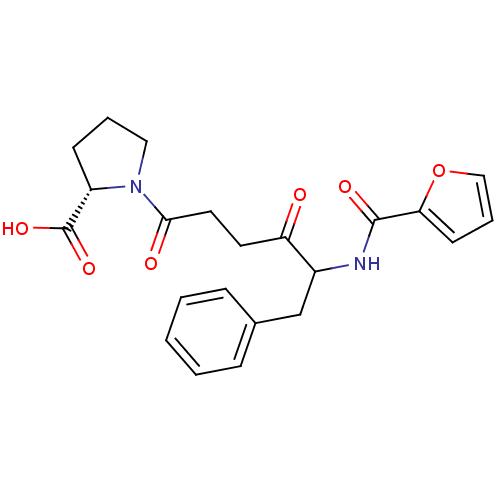
(1-{5-[(Furan-2-carbonyl)-amino]-4-oxo-6-phenyl-hex...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)c1ccco1 Show InChI InChI=1S/C22H24N2O6/c25-18(10-11-20(26)24-12-4-8-17(24)22(28)29)16(14-15-6-2-1-3-7-15)23-21(27)19-9-5-13-30-19/h1-3,5-7,9,13,16-17H,4,8,10-12,14H2,(H,23,27)(H,28,29)/t16?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027344
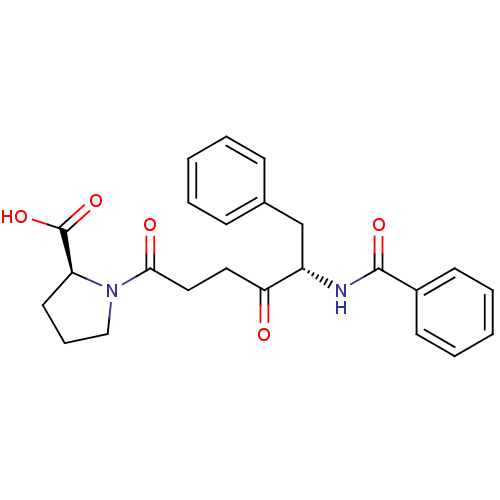
((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027344

((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of Angiotensin I converting enzyme |
J Med Chem 25: 996-9 (1982)
BindingDB Entry DOI: 10.7270/Q2154G1D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027476

(1-[2-(3-Benzoylamino-2-oxo-4-phenyl-butoxy)-acetyl...)Show SMILES OC(=O)[C@H]1CCCN1C(=O)COCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O6/c27-21(15-32-16-22(28)26-13-7-12-20(26)24(30)31)19(14-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of Angiotensin I converting enzyme |
J Med Chem 25: 996-9 (1982)
BindingDB Entry DOI: 10.7270/Q2154G1D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027147

(1-[5-Benzoylamino-6-(4-hydroxy-phenyl)-4-oxo-hexan...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccc(O)cc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O6/c27-18-10-8-16(9-11-18)15-19(25-23(30)17-5-2-1-3-6-17)21(28)12-13-22(29)26-14-4-7-20(26)24(31)32/h1-3,5-6,8-11,19-20,27H,4,7,12-15H2,(H,25,30)(H,31,32)/t19?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027144
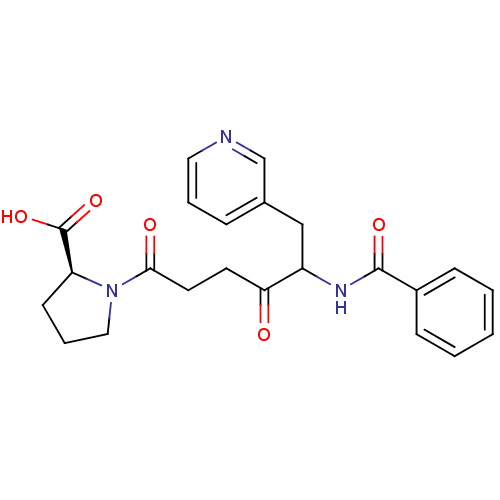
(1-(5-Benzoylamino-4-oxo-6-pyridin-3-yl-hexanoyl)-p...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1cccnc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C23H25N3O5/c27-20(10-11-21(28)26-13-5-9-19(26)23(30)31)18(14-16-6-4-12-24-15-16)25-22(29)17-7-2-1-3-8-17/h1-4,6-8,12,15,18-19H,5,9-11,13-14H2,(H,25,29)(H,30,31)/t18?,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027146
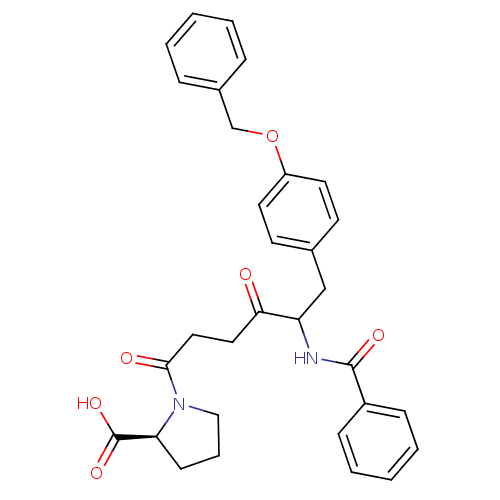
(1-[5-Benzoylamino-6-(4-benzyloxy-phenyl)-4-oxo-hex...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C31H32N2O6/c34-28(17-18-29(35)33-19-7-12-27(33)31(37)38)26(32-30(36)24-10-5-2-6-11-24)20-22-13-15-25(16-14-22)39-21-23-8-3-1-4-9-23/h1-6,8-11,13-16,26-27H,7,12,17-21H2,(H,32,36)(H,37,38)/t26?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027478

(1-[2-(3-Benzoylamino-2-oxo-4-phenyl-butylsulfanyl)...)Show SMILES OC(=O)[C@H]1CCCN1C(=O)CSCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5S/c27-21(15-32-16-22(28)26-13-7-12-20(26)24(30)31)19(14-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of Angiotensin I converting enzyme |
J Med Chem 25: 996-9 (1982)
BindingDB Entry DOI: 10.7270/Q2154G1D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of Angiotensin I converting enzyme |
J Med Chem 24: 104-9 (1981)
BindingDB Entry DOI: 10.7270/Q2ZW1MGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50286721
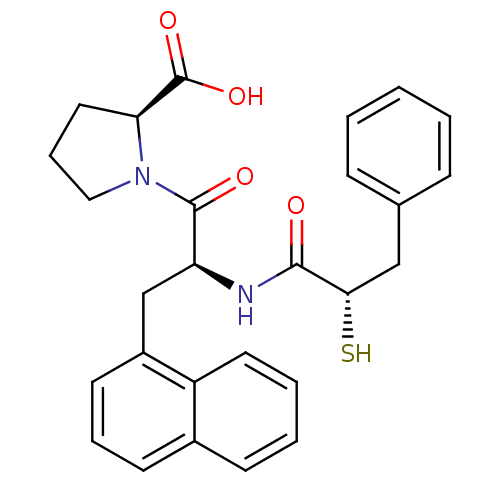
((S)-1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylami...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C27H28N2O4S/c30-25(24(34)16-18-8-2-1-3-9-18)28-22(26(31)29-15-7-14-23(29)27(32)33)17-20-12-6-11-19-10-4-5-13-21(19)20/h1-6,8-13,22-24,34H,7,14-17H2,(H,28,30)(H,32,33)/t22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 735-738 (1995)
Article DOI: 10.1016/0960-894X(95)00105-3
BindingDB Entry DOI: 10.7270/Q2611097 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027154
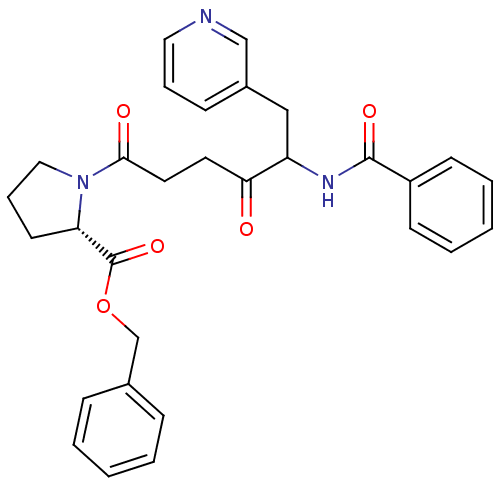
(1-(5-Benzoylamino-4-oxo-6-pyridin-3-yl-hexanoyl)-p...)Show SMILES O=C(CCC(=O)N1CCC[C@H]1C(=O)OCc1ccccc1)C(Cc1cccnc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H31N3O5/c34-27(25(19-23-11-7-17-31-20-23)32-29(36)24-12-5-2-6-13-24)15-16-28(35)33-18-8-14-26(33)30(37)38-21-22-9-3-1-4-10-22/h1-7,9-13,17,20,25-26H,8,14-16,18-19,21H2,(H,32,36)/t25?,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50286722
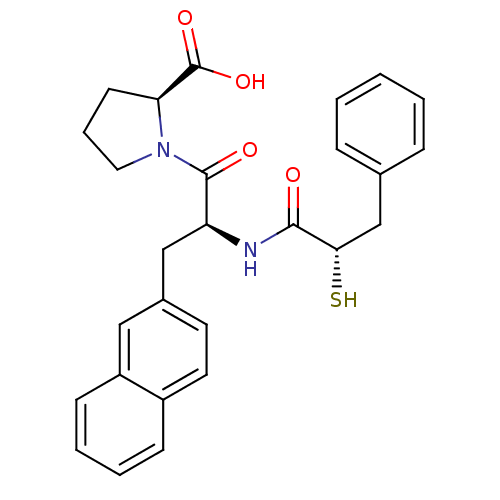
((S)-1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylami...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C27H28N2O4S/c30-25(24(34)17-18-7-2-1-3-8-18)28-22(26(31)29-14-6-11-23(29)27(32)33)16-19-12-13-20-9-4-5-10-21(20)15-19/h1-5,7-10,12-13,15,22-24,34H,6,11,14,16-17H2,(H,28,30)(H,32,33)/t22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 735-738 (1995)
Article DOI: 10.1016/0960-894X(95)00105-3
BindingDB Entry DOI: 10.7270/Q2611097 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027133

(1-{4-Oxo-6-phenyl-5-[(tetrahydro-furan-2-carbonyl)...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)C1CCCO1 Show InChI InChI=1S/C22H28N2O6/c25-18(10-11-20(26)24-12-4-8-17(24)22(28)29)16(14-15-6-2-1-3-7-15)23-21(27)19-9-5-13-30-19/h1-3,6-7,16-17,19H,4-5,8-14H2,(H,23,27)(H,28,29)/t16?,17-,19?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283658
((S)-3-Biphenyl-4-yl-2-[(S)-2-((S)-1-carboxy-ethyla...)Show SMILES CC[C@H](C)C(N[C@@H](C)C(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C24H30N2O5/c1-4-15(2)21(25-16(3)23(28)29)22(27)26-20(24(30)31)14-17-10-12-19(13-11-17)18-8-6-5-7-9-18/h5-13,15-16,20-21,25H,4,14H2,1-3H3,(H,26,27)(H,28,29)(H,30,31)/t15-,16-,20-,21?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283656
((S)-3-Biphenyl-4-yl-2-[(S)-2-((S)-1-carboxy-ethyla...)Show SMILES CC(C)[C@H](N[C@@H](C)C(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C23H28N2O5/c1-14(2)20(24-15(3)22(27)28)21(26)25-19(23(29)30)13-16-9-11-18(12-10-16)17-7-5-4-6-8-17/h4-12,14-15,19-20,24H,13H2,1-3H3,(H,25,26)(H,27,28)(H,29,30)/t15-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition against angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 4: 2673-2676 (1994)
Article DOI: 10.1016/S0960-894X(01)80694-6
BindingDB Entry DOI: 10.7270/Q2X34XXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50085459
((S)-3-Biphenyl-4-yl-2-[(S)-3-methyl-2-(phosphonome...)Show SMILES CC(C)[C@H](NCP(O)(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H27N2O6P/c1-14(2)19(22-13-30(27,28)29)20(24)23-18(21(25)26)12-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,22H,12-13H2,1-2H3,(H,23,24)(H,25,26)(H2,27,28,29)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme in Hog plasma |
J Med Chem 33: 1606-15 (1990)
BindingDB Entry DOI: 10.7270/Q2CF9QPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50286723

((S)-1-[(S)-2-((R)-2-Mercapto-3-phenyl-propionylami...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H](S)Cc1ccccc1 Show InChI InChI=1S/C27H28N2O4S/c30-25(24(34)16-18-8-2-1-3-9-18)28-22(26(31)29-15-7-14-23(29)27(32)33)17-20-12-6-11-19-10-4-5-13-21(19)20/h1-6,8-13,22-24,34H,7,14-17H2,(H,28,30)(H,32,33)/t22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 735-738 (1995)
Article DOI: 10.1016/0960-894X(95)00105-3
BindingDB Entry DOI: 10.7270/Q2611097 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027152
(2-(5-Benzoylamino-4-oxo-6-phenyl-hexanoylamino)-3-...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)CCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C28H28N2O5/c31-25(16-17-26(32)29-24(28(34)35)19-21-12-6-2-7-13-21)23(18-20-10-4-1-5-11-20)30-27(33)22-14-8-3-9-15-22/h1-15,23-24H,16-19H2,(H,29,32)(H,30,33)(H,34,35)/t23?,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288346
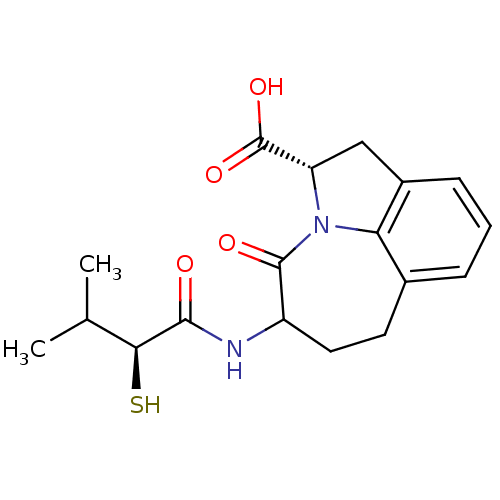
((S)-5-((S)-2-Mercapto-3-methyl-butyrylamino)-4-oxo...)Show SMILES CC(C)[C@H](S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C18H22N2O4S/c1-9(2)15(25)16(21)19-12-7-6-10-4-3-5-11-8-13(18(23)24)20(14(10)11)17(12)22/h3-5,9,12-13,15,25H,6-8H2,1-2H3,(H,19,21)(H,23,24)/t12?,13-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50403594
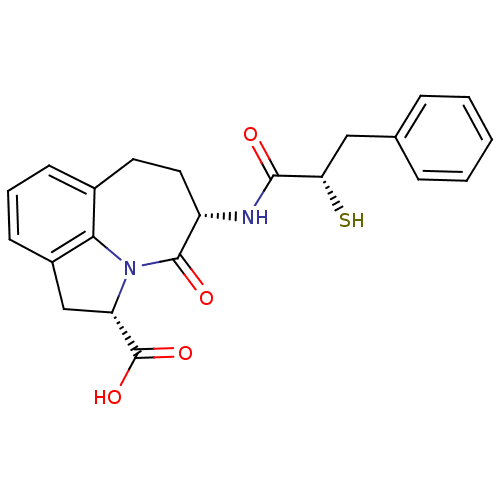
(CHEMBL2112403)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)[C@@H](S)Cc4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C22H22N2O4S/c25-20(18(29)11-13-5-2-1-3-6-13)23-16-10-9-14-7-4-8-15-12-17(22(27)28)24(19(14)15)21(16)26/h1-8,16-18,29H,9-12H2,(H,23,25)(H,27,28)/t16-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288349
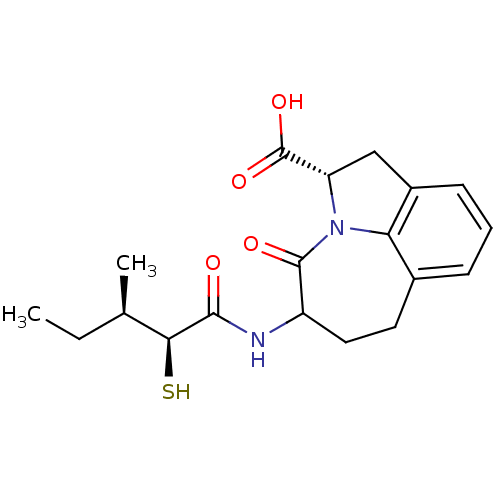
((S)-5-((2S,3R)-2-Mercapto-3-methyl-pentanoylamino)...)Show SMILES CC[C@@H](C)[C@H](S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C19H24N2O4S/c1-3-10(2)16(26)17(22)20-13-8-7-11-5-4-6-12-9-14(19(24)25)21(15(11)12)18(13)23/h4-6,10,13-14,16,26H,3,7-9H2,1-2H3,(H,20,22)(H,24,25)/t10-,13?,14+,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027480
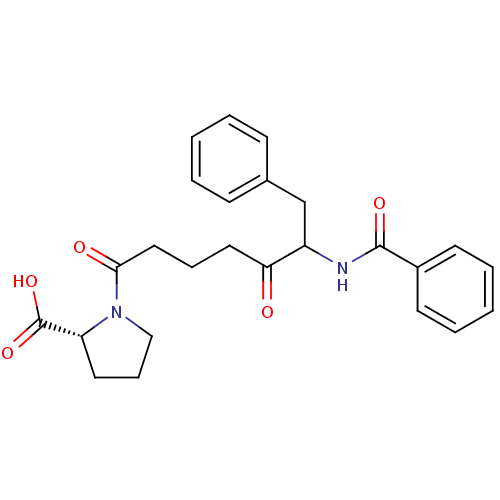
(1-(6-Benzoylamino-5-oxo-7-phenyl-heptanoyl)-pyrrol...)Show SMILES OC(=O)[C@H]1CCCN1C(=O)CCCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C25H28N2O5/c28-22(14-7-15-23(29)27-16-8-13-21(27)25(31)32)20(17-18-9-3-1-4-10-18)26-24(30)19-11-5-2-6-12-19/h1-6,9-12,20-21H,7-8,13-17H2,(H,26,30)(H,31,32)/t20?,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of Angiotensin I converting enzyme |
J Med Chem 25: 996-9 (1982)
BindingDB Entry DOI: 10.7270/Q2154G1D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027153
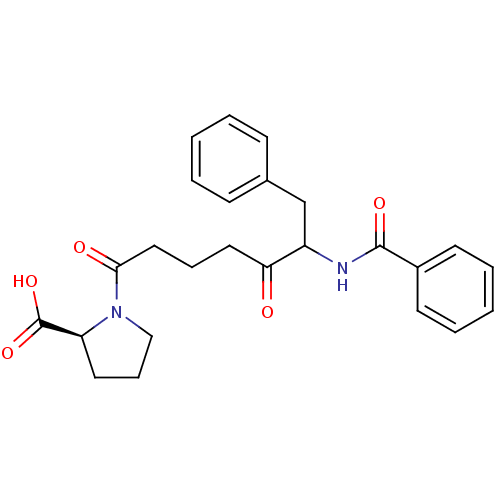
(1-(6-Benzoylamino-5-oxo-7-phenyl-heptanoyl)-pyrrol...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C25H28N2O5/c28-22(14-7-15-23(29)27-16-8-13-21(27)25(31)32)20(17-18-9-3-1-4-10-18)26-24(30)19-11-5-2-6-12-19/h1-6,9-12,20-21H,7-8,13-17H2,(H,26,30)(H,31,32)/t20?,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283644
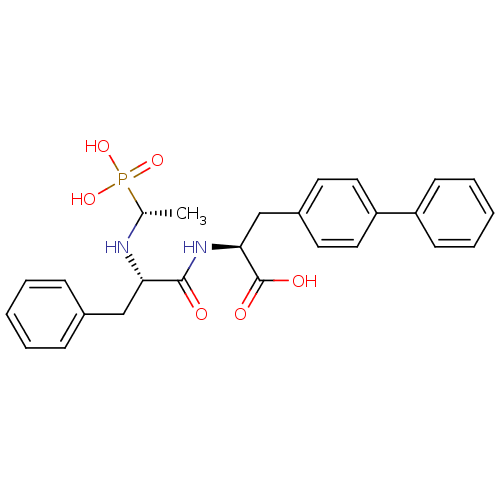
((S)-3-Biphenyl-4-yl-2-[(S)-3-phenyl-2-((R)-1-phosp...)Show SMILES C[C@H](N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O)P(O)(O)=O Show InChI InChI=1S/C26H29N2O6P/c1-18(35(32,33)34)27-23(16-19-8-4-2-5-9-19)25(29)28-24(26(30)31)17-20-12-14-22(15-13-20)21-10-6-3-7-11-21/h2-15,18,23-24,27H,16-17H2,1H3,(H,28,29)(H,30,31)(H2,32,33,34)/t18-,23+,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027150
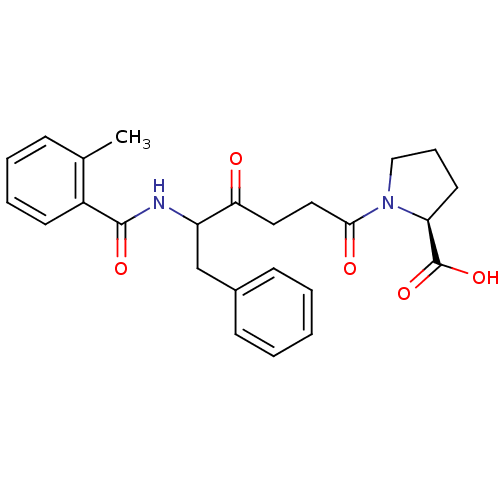
(1-[5-(2-Methyl-benzoylamino)-4-oxo-6-phenyl-hexano...)Show SMILES Cc1ccccc1C(=O)NC(Cc1ccccc1)C(=O)CCC(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C25H28N2O5/c1-17-8-5-6-11-19(17)24(30)26-20(16-18-9-3-2-4-10-18)22(28)13-14-23(29)27-15-7-12-21(27)25(31)32/h2-6,8-11,20-21H,7,12-16H2,1H3,(H,26,30)(H,31,32)/t20?,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283642
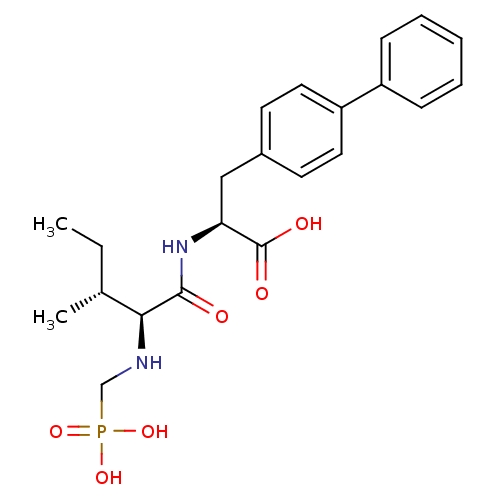
((S)-3-Biphenyl-4-yl-2-{(S)-3-methyl-2-[((R)-phosph...)Show SMILES CC[C@@H](C)[C@H](NCP(O)(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C22H29N2O6P/c1-3-15(2)20(23-14-31(28,29)30)21(25)24-19(22(26)27)13-16-9-11-18(12-10-16)17-7-5-4-6-8-17/h4-12,15,19-20,23H,3,13-14H2,1-2H3,(H,24,25)(H,26,27)(H2,28,29,30)/t15-,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283639
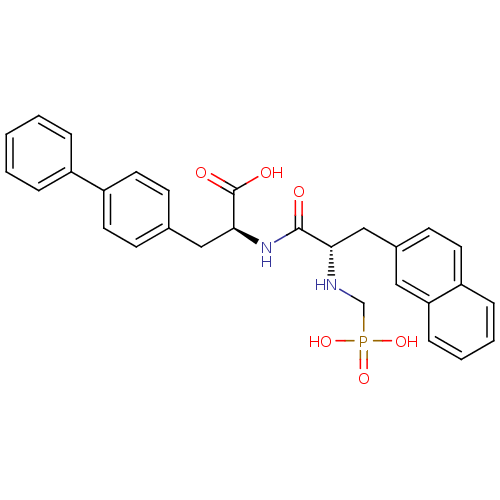
((S)-3-Biphenyl-4-yl-2-[(S)-3-naphthalen-2-yl-2-(ph...)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)NCP(O)(O)=O Show InChI InChI=1S/C29H29N2O6P/c32-28(26(30-19-38(35,36)37)18-21-12-15-23-8-4-5-9-25(23)16-21)31-27(29(33)34)17-20-10-13-24(14-11-20)22-6-2-1-3-7-22/h1-16,26-27,30H,17-19H2,(H,31,32)(H,33,34)(H2,35,36,37)/t26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50286720
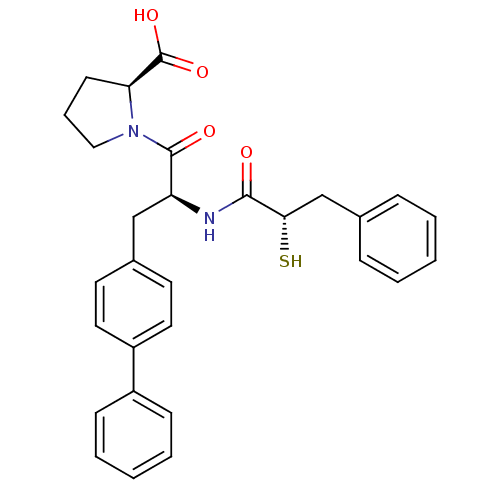
((S)-1-[(S)-3-Biphenyl-4-yl-2-((S)-2-mercapto-3-phe...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C29H30N2O4S/c32-27(26(36)19-20-8-3-1-4-9-20)30-24(28(33)31-17-7-12-25(31)29(34)35)18-21-13-15-23(16-14-21)22-10-5-2-6-11-22/h1-6,8-11,13-16,24-26,36H,7,12,17-19H2,(H,30,32)(H,34,35)/t24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 735-738 (1995)
Article DOI: 10.1016/0960-894X(95)00105-3
BindingDB Entry DOI: 10.7270/Q2611097 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50048306
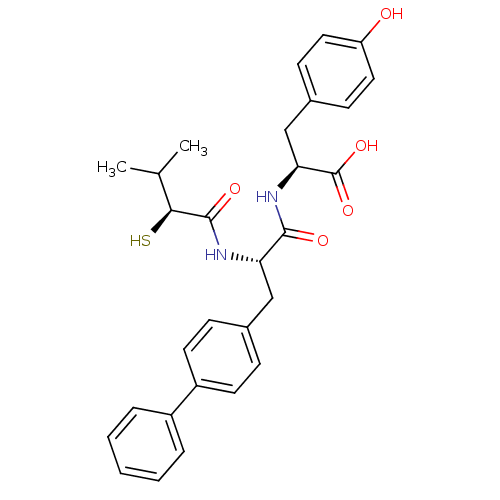
((S)-2-[(S)-3-Biphenyl-4-yl-2-((S)-2-mercapto-3-met...)Show SMILES CC(C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C29H32N2O5S/c1-18(2)26(37)28(34)30-24(16-19-8-12-22(13-9-19)21-6-4-3-5-7-21)27(33)31-25(29(35)36)17-20-10-14-23(32)15-11-20/h3-15,18,24-26,32,37H,16-17H2,1-2H3,(H,30,34)(H,31,33)(H,35,36)/t24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 735-738 (1995)
Article DOI: 10.1016/0960-894X(95)00105-3
BindingDB Entry DOI: 10.7270/Q2611097 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50403593

(CHEMBL2111596)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@@H](NC(=O)[C@@H](S)Cc4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C22H22N2O4S/c25-20(18(29)11-13-5-2-1-3-6-13)23-16-10-9-14-7-4-8-15-12-17(22(27)28)24(19(14)15)21(16)26/h1-8,16-18,29H,9-12H2,(H,23,25)(H,27,28)/t16-,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283660
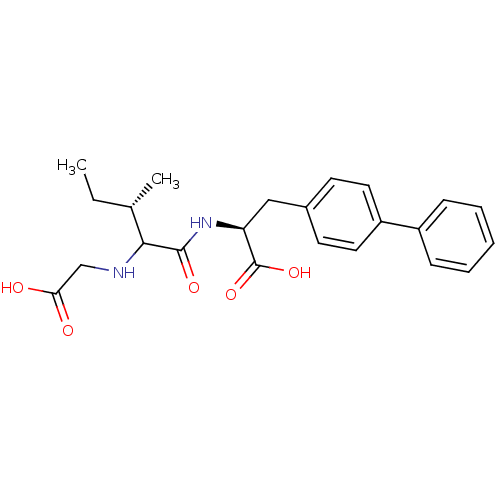
((S)-3-Biphenyl-4-yl-2-[(S)-2-(carboxymethyl-amino)...)Show SMILES CC[C@H](C)C(NCC(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C23H28N2O5/c1-3-15(2)21(24-14-20(26)27)22(28)25-19(23(29)30)13-16-9-11-18(12-10-16)17-7-5-4-6-8-17/h4-12,15,19,21,24H,3,13-14H2,1-2H3,(H,25,28)(H,26,27)(H,29,30)/t15-,19-,21?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50285934
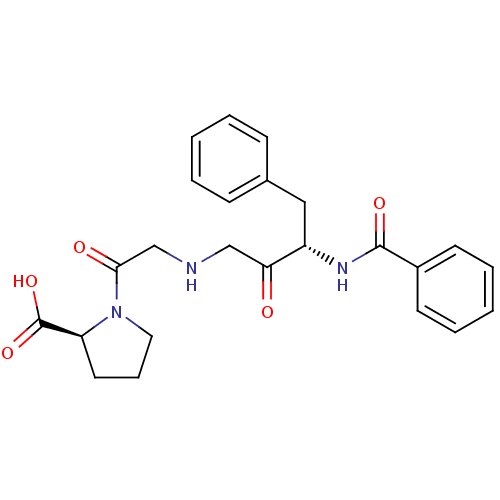
(1-[2-((S)-3-Benzoylamino-2-oxo-4-phenyl-butylamino...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CNCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H27N3O5/c28-21(15-25-16-22(29)27-13-7-12-20(27)24(31)32)19(14-17-8-3-1-4-9-17)26-23(30)18-10-5-2-6-11-18/h1-6,8-11,19-20,25H,7,12-16H2,(H,26,30)(H,31,32)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme |
Bioorg Med Chem Lett 5: 2825-2828 (1995)
Article DOI: 10.1016/0960-894X(95)00494-E
BindingDB Entry DOI: 10.7270/Q2JM29KW |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50403595
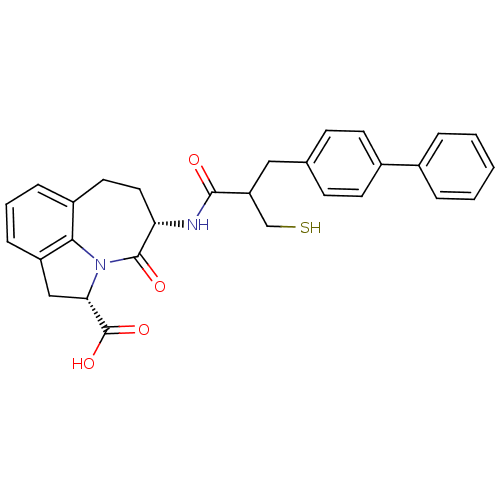
(CHEMBL2112402)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)C(CS)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C29H28N2O4S/c32-27(23(17-36)15-18-9-11-20(12-10-18)19-5-2-1-3-6-19)30-24-14-13-21-7-4-8-22-16-25(29(34)35)31(26(21)22)28(24)33/h1-12,23-25,36H,13-17H2,(H,30,32)(H,34,35)/t23?,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50403595

(CHEMBL2112402)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)C(CS)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C29H28N2O4S/c32-27(23(17-36)15-18-9-11-20(12-10-18)19-5-2-1-3-6-19)30-24-14-13-21-7-4-8-22-16-25(29(34)35)31(26(21)22)28(24)33/h1-12,23-25,36H,13-17H2,(H,30,32)(H,34,35)/t23?,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50286715
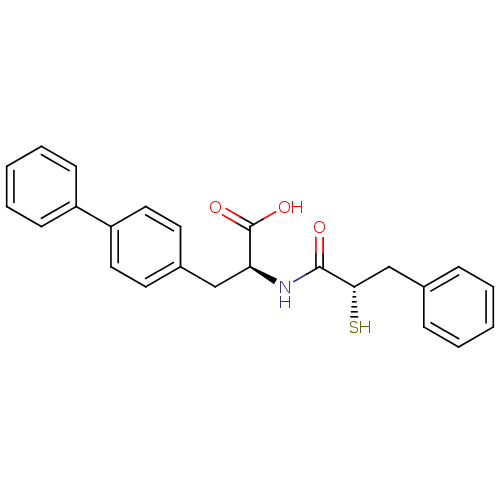
((S)-3-biphenyl-4-yl-2-((S)-2-mercapto-3-phenyl-pro...)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C24H23NO3S/c26-23(22(29)16-17-7-3-1-4-8-17)25-21(24(27)28)15-18-11-13-20(14-12-18)19-9-5-2-6-10-19/h1-14,21-22,29H,15-16H2,(H,25,26)(H,27,28)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 735-738 (1995)
Article DOI: 10.1016/0960-894X(95)00105-3
BindingDB Entry DOI: 10.7270/Q2611097 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027148
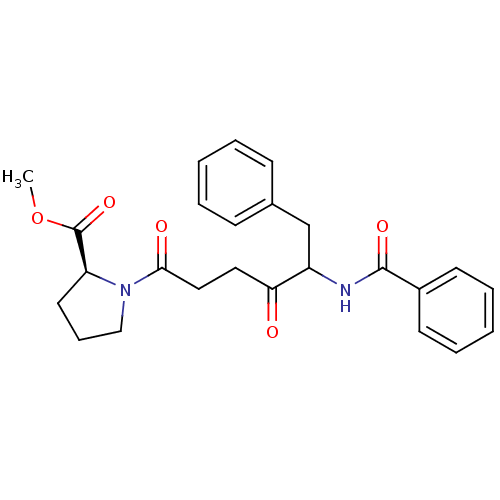
(1-(5-Benzoylamino-4-oxo-6-phenyl-hexanoyl)-pyrroli...)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C25H28N2O5/c1-32-25(31)21-13-8-16-27(21)23(29)15-14-22(28)20(17-18-9-4-2-5-10-18)26-24(30)19-11-6-3-7-12-19/h2-7,9-12,20-21H,8,13-17H2,1H3,(H,26,30)/t20?,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027140
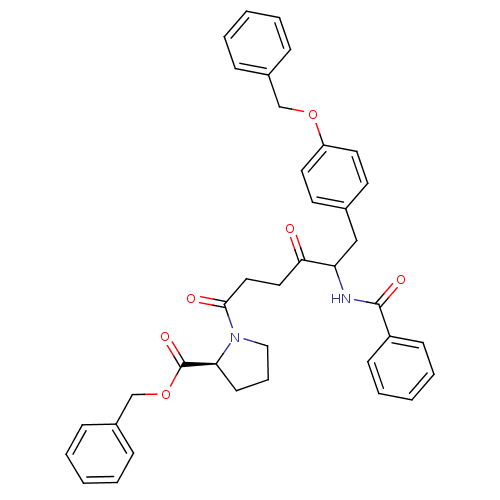
(1-[5-Benzoylamino-6-(4-benzyloxy-phenyl)-4-oxo-hex...)Show SMILES O=C(CCC(=O)N1CCC[C@H]1C(=O)OCc1ccccc1)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C38H38N2O6/c41-35(22-23-36(42)40-24-10-17-34(40)38(44)46-27-30-13-6-2-7-14-30)33(39-37(43)31-15-8-3-9-16-31)25-28-18-20-32(21-19-28)45-26-29-11-4-1-5-12-29/h1-9,11-16,18-21,33-34H,10,17,22-27H2,(H,39,43)/t33?,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288354

((S)-5-((S)-3-Biphenyl-4-yl-2-mercapto-propionylami...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)[C@@H](S)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C28H26N2O4S/c31-26(24(35)15-17-9-11-19(12-10-17)18-5-2-1-3-6-18)29-22-14-13-20-7-4-8-21-16-23(28(33)34)30(25(20)21)27(22)32/h1-12,22-24,35H,13-16H2,(H,29,31)(H,33,34)/t22?,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283640
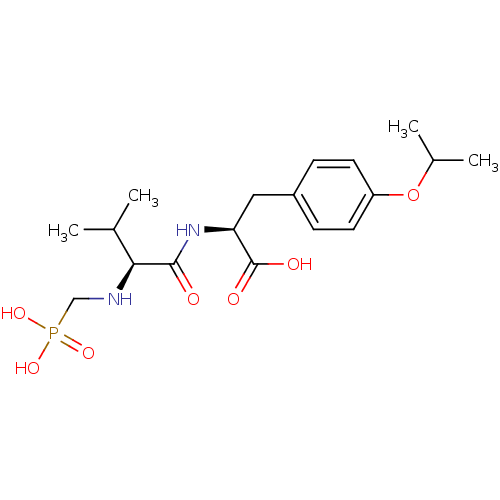
((S)-3-(4-Isopropoxy-phenyl)-2-[(S)-3-methyl-2-(pho...)Show SMILES CC(C)Oc1ccc(C[C@H](NC(=O)[C@@H](NCP(O)(O)=O)C(C)C)C(O)=O)cc1 Show InChI InChI=1S/C18H29N2O7P/c1-11(2)16(19-10-28(24,25)26)17(21)20-15(18(22)23)9-13-5-7-14(8-6-13)27-12(3)4/h5-8,11-12,15-16,19H,9-10H2,1-4H3,(H,20,21)(H,22,23)(H2,24,25,26)/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288348

((S)-5-((S)-2-Cyclohexyl-2-mercapto-acetylamino)-4-...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)[C@@H](S)C4CCCCC4)C(=O)N1c23 Show InChI InChI=1S/C21H26N2O4S/c24-19(18(28)13-5-2-1-3-6-13)22-15-10-9-12-7-4-8-14-11-16(21(26)27)23(17(12)14)20(15)25/h4,7-8,13,15-16,18,28H,1-3,5-6,9-11H2,(H,22,24)(H,26,27)/t15?,16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283648
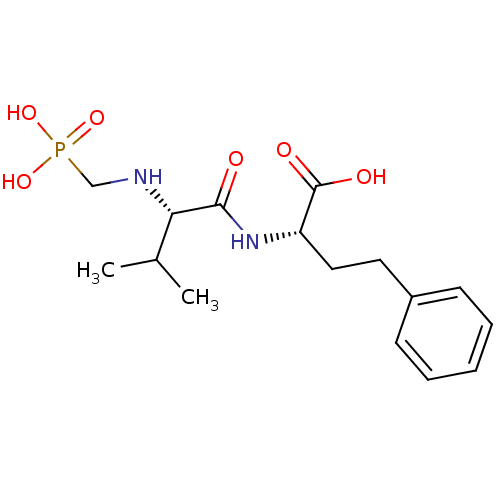
((S)-2-[(S)-3-Methyl-2-(phosphonomethyl-amino)-buty...)Show SMILES CC(C)[C@H](NCP(O)(O)=O)C(=O)N[C@@H](CCc1ccccc1)C(O)=O Show InChI InChI=1S/C16H25N2O6P/c1-11(2)14(17-10-25(22,23)24)15(19)18-13(16(20)21)9-8-12-6-4-3-5-7-12/h3-7,11,13-14,17H,8-10H2,1-2H3,(H,18,19)(H,20,21)(H2,22,23,24)/t13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50286718
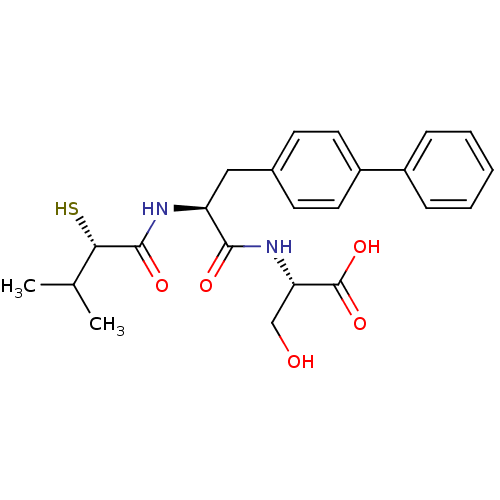
((S)-2-[(S)-3-Biphenyl-4-yl-2-((S)-2-mercapto-3-met...)Show SMILES CC(C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CO)C(O)=O Show InChI InChI=1S/C23H28N2O5S/c1-14(2)20(31)22(28)24-18(21(27)25-19(13-26)23(29)30)12-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-20,26,31H,12-13H2,1-2H3,(H,24,28)(H,25,27)(H,29,30)/t18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 735-738 (1995)
Article DOI: 10.1016/0960-894X(95)00105-3
BindingDB Entry DOI: 10.7270/Q2611097 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50283652
((S)-3-Biphenyl-4-yl-2-[(S)-3-phenyl-2-(phosphonome...)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](Cc1ccccc1)NCP(O)(O)=O Show InChI InChI=1S/C25H27N2O6P/c28-24(22(26-17-34(31,32)33)15-18-7-3-1-4-8-18)27-23(25(29)30)16-19-11-13-21(14-12-19)20-9-5-2-6-10-20/h1-14,22-23,26H,15-17H2,(H,27,28)(H,29,30)(H2,31,32,33)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
Bioorg Med Chem Lett 4: 2715-2720 (1994)
Article DOI: 10.1016/S0960-894X(01)80703-4
BindingDB Entry DOI: 10.7270/Q2SB467B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data