

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50472361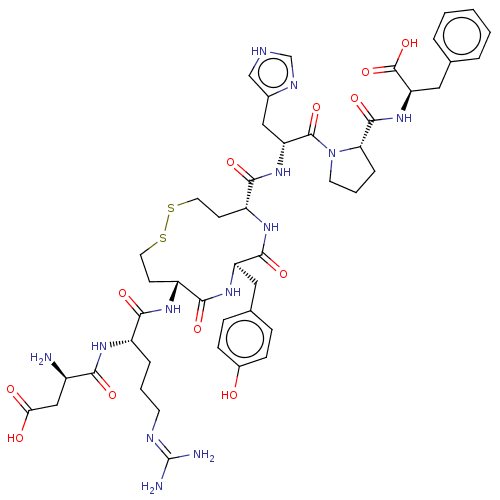 (CHEMBL406349) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor, type 1 from rat liver membranes | J Med Chem 42: 4524-37 (1999) Article DOI: 10.1021/jm991089q BindingDB Entry DOI: 10.7270/Q2PN98C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228195 (Angiotensin Ii | CHEBI:2719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor, type 1 from rat liver membranes | J Med Chem 42: 4524-37 (1999) Article DOI: 10.1021/jm991089q BindingDB Entry DOI: 10.7270/Q2PN98C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50212596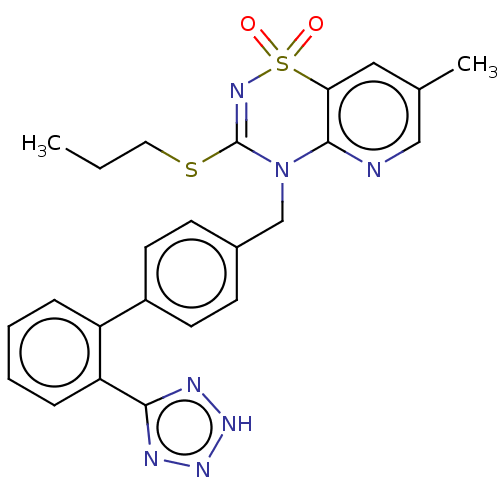 (CHEMBL13813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding against Angiotensin II receptor in a radioligand binding assay using [125I]-Sar1-Ile8 angiotensin-II as radioligand in rat adrenal cortical m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C53P05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125339 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50212594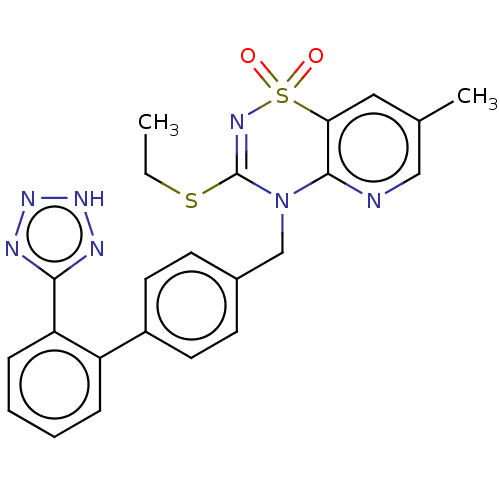 (CHEMBL13783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding against Angiotensin II receptor in a radioligand binding assay using [125I]-Sar1-Ile8 angiotensin-II as radioligand in rat adrenal cortical m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C53P05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125362 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125352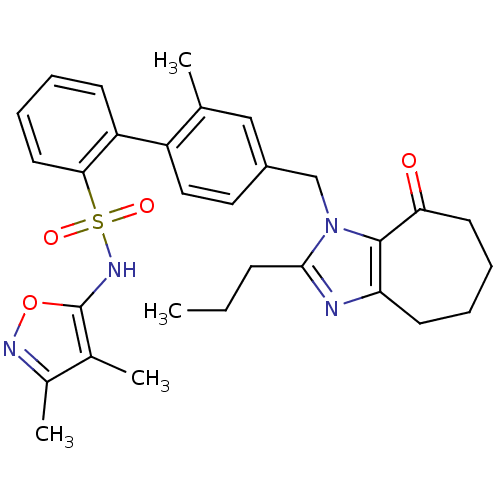 (2'-Methyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahydro-4H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125356 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-(2-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125359 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470323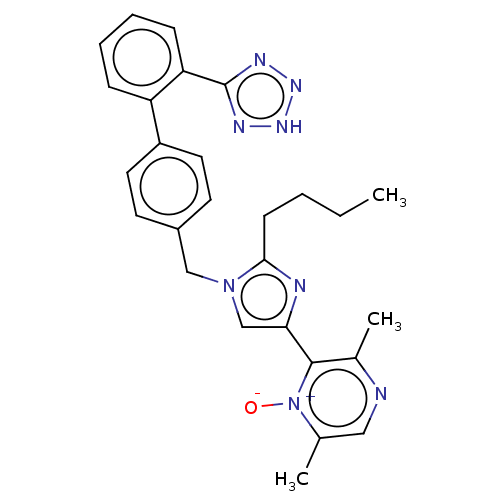 (CHEMBL327505) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125350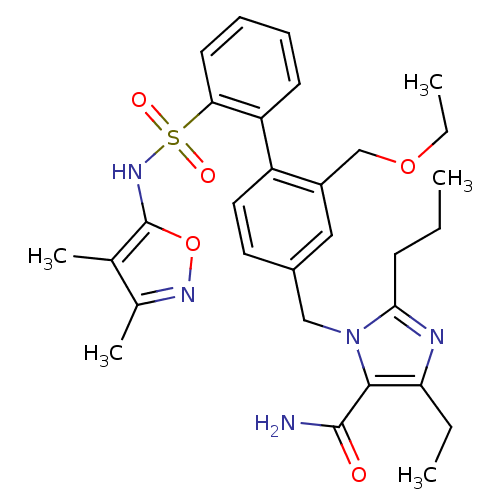 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-etho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125340 (2'-Methoxymethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125345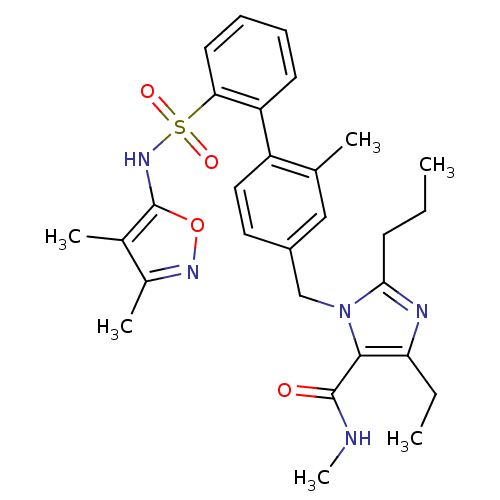 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470328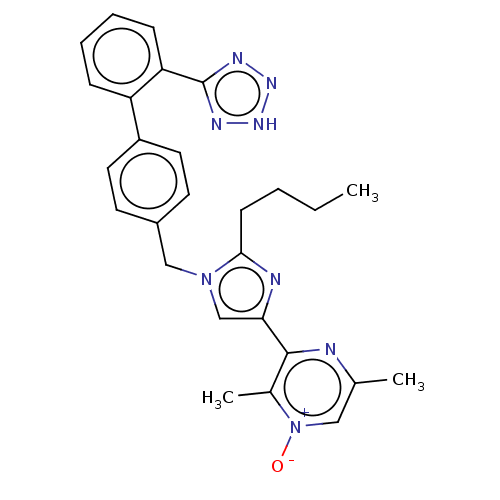 (CHEMBL317300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470310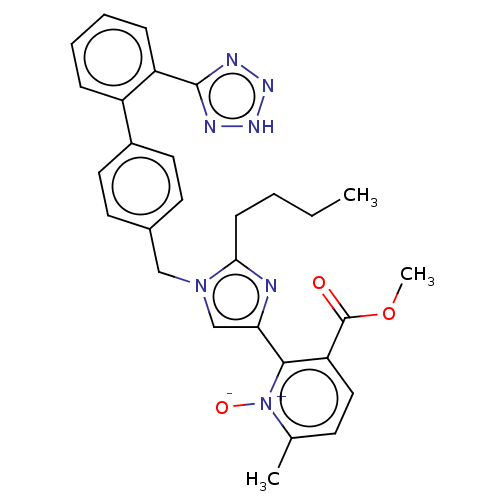 (CHEMBL98592) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231140 (CHEMBL77029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50212600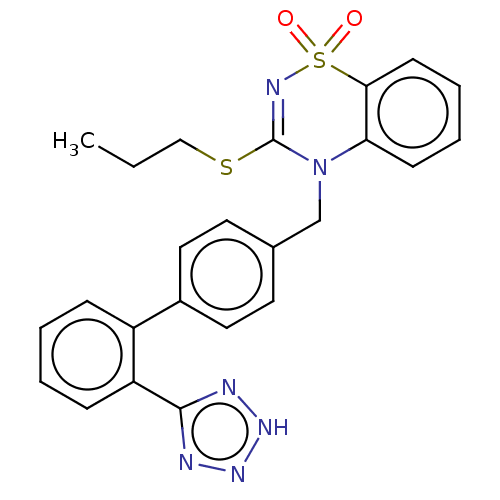 (CHEMBL274887) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding against Angiotensin II receptor in a radioligand binding assay using [125I]-Sar1-Ile8 angiotensin-II as radioligand in rat adrenal cortical m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C53P05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125360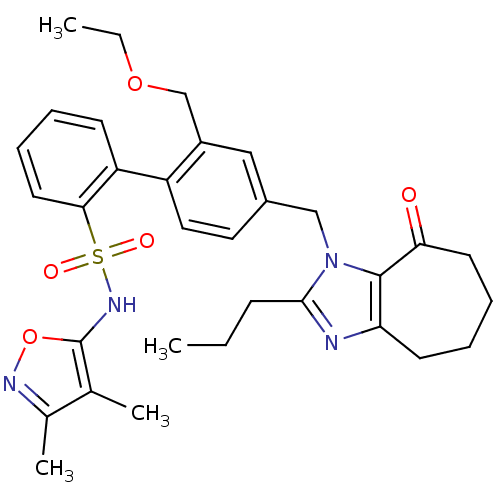 (2'-Ethoxymethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145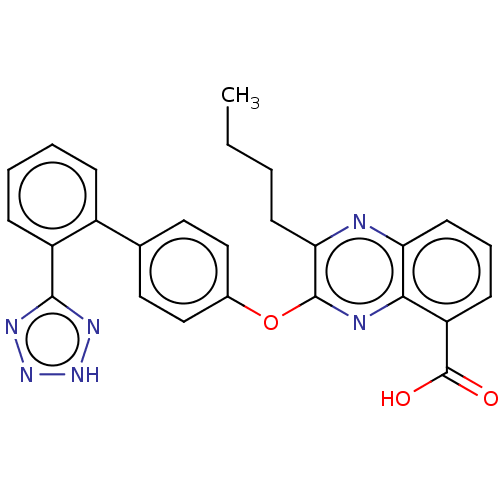 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding against Angiotensin II receptor in a radioligand binding assay using [125I]-Sar1-Ile8 angiotensin-II as radioligand in rat adrenal cortical m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C53P05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50117910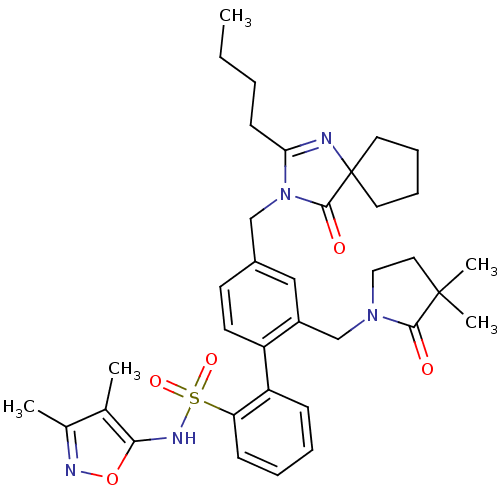 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125358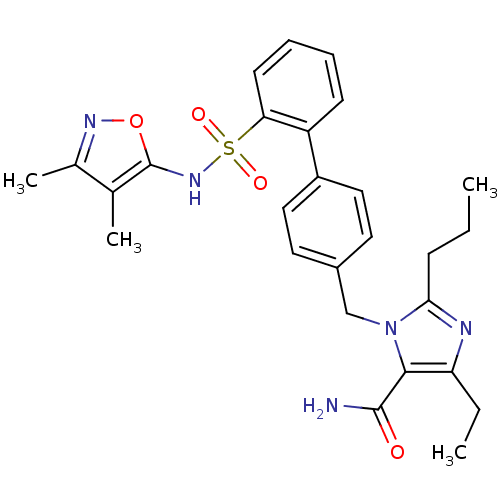 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231150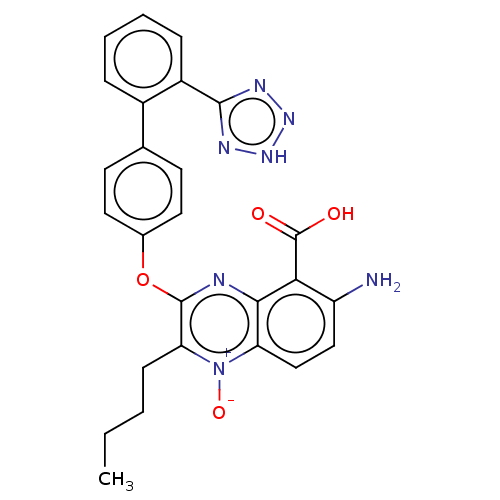 (CHEMBL77935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470321 (CHEMBL99008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125341 (4'-(8-Oxo-2-propyl-5,6,7,8-tetrahydro-4H-cyclohept...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125361 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50212595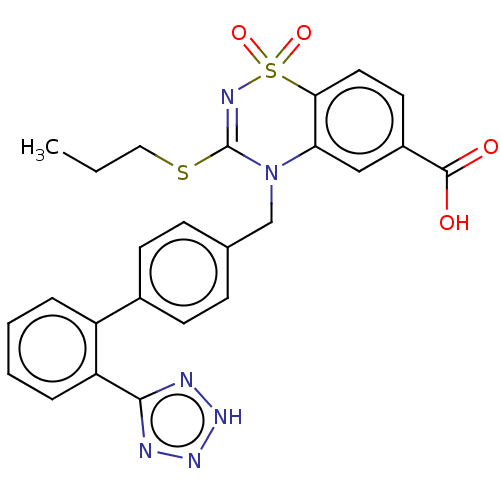 (CHEMBL268004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding against Angiotensin II receptor in a radioligand binding assay using [125I]-Sar1-Ile8 angiotensin-II as radioligand in rat adrenal cortical m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C53P05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470340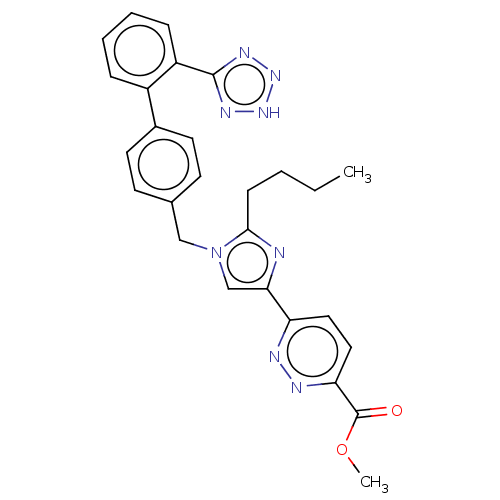 (CHEMBL98370) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231139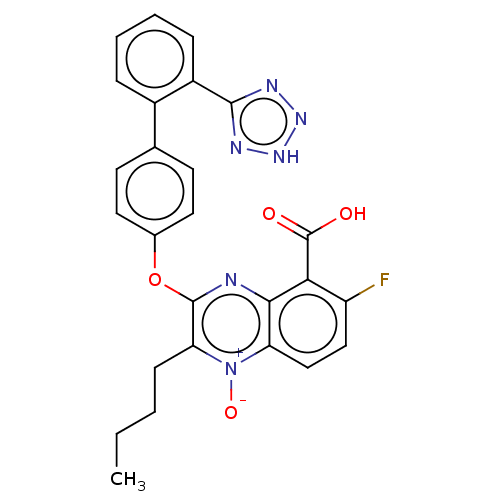 (CHEMBL77827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470326 (CHEMBL319990) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470313 (CHEMBL441640) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470330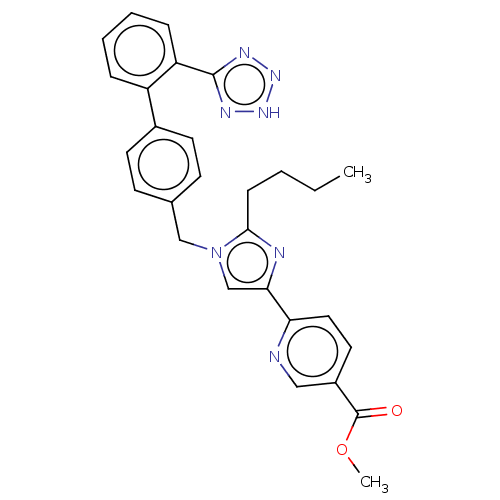 (CHEMBL318194) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125344 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125348 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-etho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231149 (CHEMBL306278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125355 (2'-Fluoro-4'-(8-oxo-2-propyl-5,6,7,8-tetrahydro-4H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125354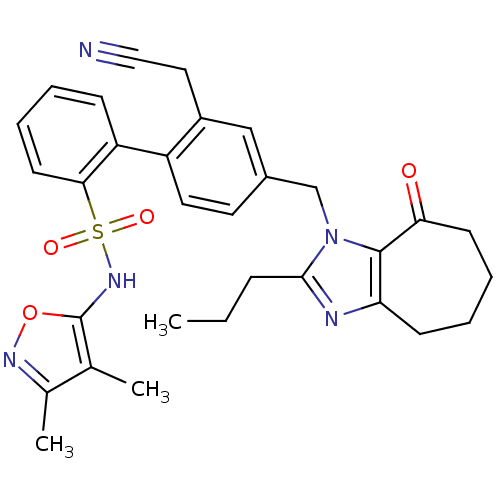 (2'-Cyanomethyl-4'-(8-oxo-2-propyl-5,6,7,8-tetrahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231143 (CHEMBL75053) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470317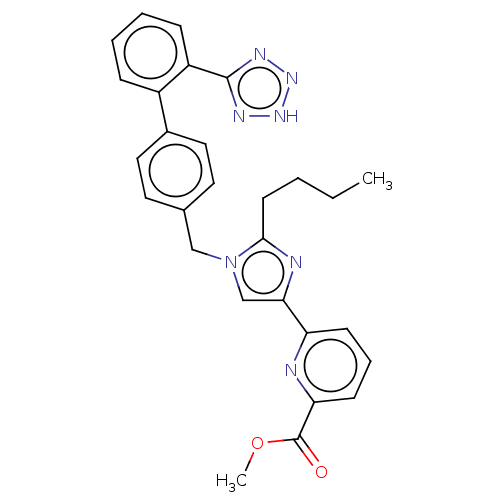 (CHEMBL319370) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125346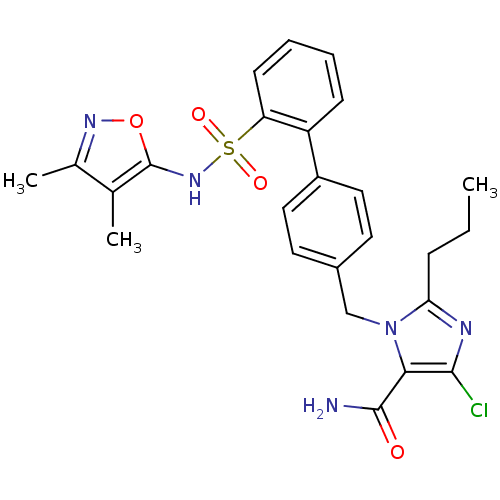 (5-Chloro-3-[2'-(3,4-dimethyl-isoxazol-5-ylsulfamoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231007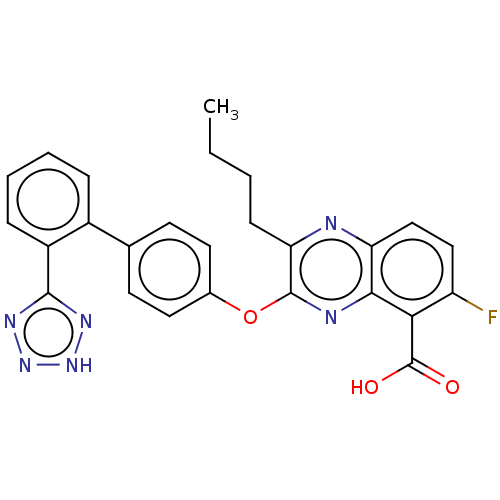 (CHEMBL74445) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231142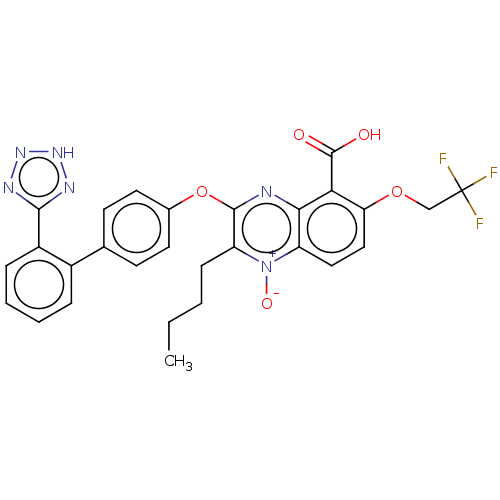 (CHEMBL312068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125349 (5-Chloro-3-[2'-(3,4-dimethyl-isoxazol-5-ylsulfamoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125357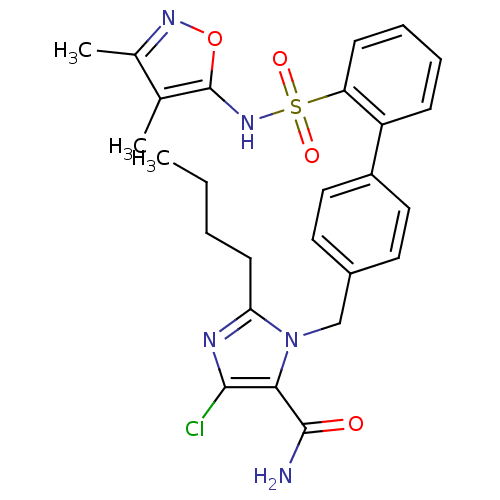 (2-Butyl-5-chloro-3-[2'-(3,4-dimethyl-isoxazol-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470309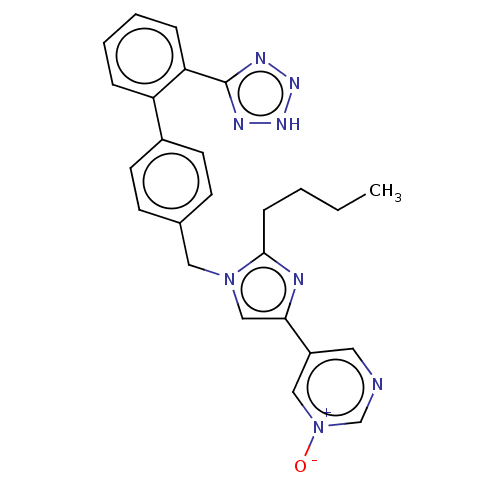 (CHEMBL318898) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470336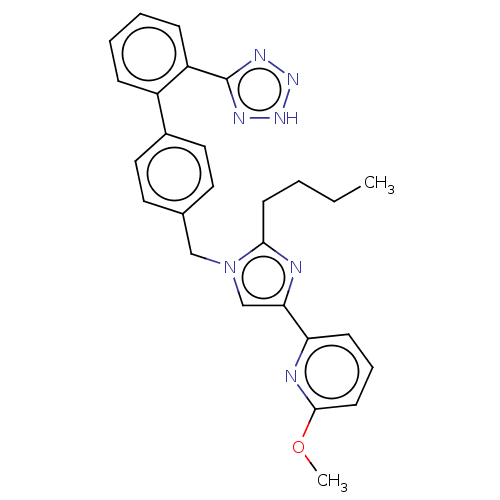 (CHEMBL317205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470338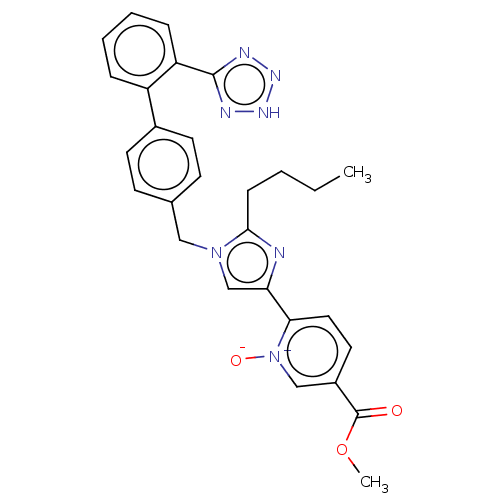 (CHEMBL101952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |