 Found 12 hits of ic50 for monomerid = 50318494
Found 12 hits of ic50 for monomerid = 50318494 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S
(Homo sapiens (Human)) | BDBM50318494
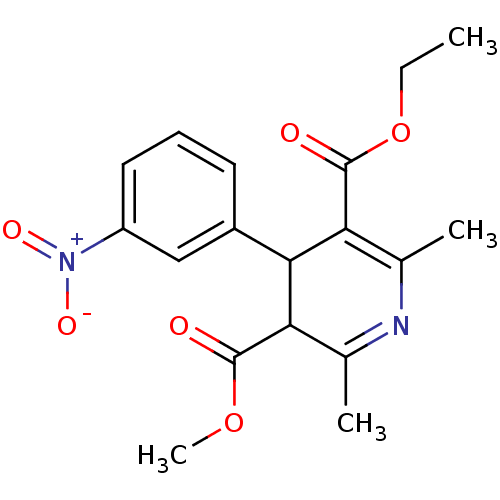
(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel of guinea pig ileal longitudinal smooth muscle |
J Med Chem 31: 1489-92 (1988)
BindingDB Entry DOI: 10.7270/Q28W3GJ5 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated L-type Ca channel (species unknown) |
Cardiovasc Res 91: 53-61 (2011)
Article DOI: 10.1093/cvr/cvr044
BindingDB Entry DOI: 10.7270/Q2NP264F |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research
Curated by ChEMBL
| Assay Description
In vitro vasorelaxant activity (calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta |
J Med Chem 33: 2629-35 (1990)
BindingDB Entry DOI: 10.7270/Q2XD13XV |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 55
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
| Assay Description
Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... |
PubChem Bioassay (2010)
BindingDB Entry DOI: 10.7270/Q2348HSG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of hERG K channel |
Cardiovasc Res 91: 53-61 (2011)
Article DOI: 10.1093/cvr/cvr044
BindingDB Entry DOI: 10.7270/Q2NP264F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TCG Lifesciences Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 46: 618-30 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.042
BindingDB Entry DOI: 10.7270/Q2WQ052W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Potassium channel HERG expressed in mammalian cells |
Bioorg Med Chem Lett 13: 2773-5 (2003)
BindingDB Entry DOI: 10.7270/Q2QZ2BGZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by whole cell patch clamp technique |
Bioorg Med Chem 16: 6252-60 (2008)
Article DOI: 10.1016/j.bmc.2008.04.028
BindingDB Entry DOI: 10.7270/Q25D8T25 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Reverse proteomics research institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against potassium channel HERG |
Bioorg Med Chem Lett 15: 2886-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.080
BindingDB Entry DOI: 10.7270/Q29S1S7C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of Na channel (species unknown) |
Cardiovasc Res 91: 53-61 (2011)
Article DOI: 10.1093/cvr/cvr044
BindingDB Entry DOI: 10.7270/Q2NP264F |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea... |
J Med Chem 28: 381-8 (1985)
BindingDB Entry DOI: 10.7270/Q2Z321T8 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50318494

(3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,14,16H,5H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research UK
Curated by ChEMBL
| Assay Description
Inhibition of Thromboxane A2 synthase after oral administration of 0.03 mmol/kg |
J Med Chem 33: 646-52 (1990)
BindingDB Entry DOI: 10.7270/Q25X29HP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data