

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM237182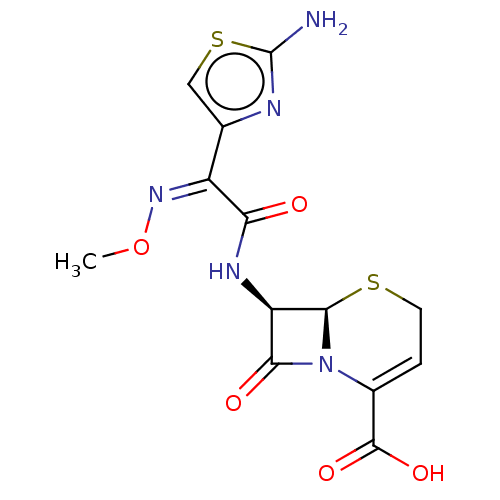 (Ceftizoxime) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | 2.28E+4 | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ondokuz Mayis University | Assay Description Enzymatic activity was measured by Beutler's method with a Shimadzu Spectrophotometer UV-(1208), at 25°C. The assay system contained 100 mM Tris-... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237182 (Ceftizoxime) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | 5.18E+4 | -24.5 | 7.91E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Erzincan University | Assay Description CA activity was assayed according to method of Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] described previously by Innocenti e... | J Enzyme Inhib Med Chem 27: 641-5 (2012) Article DOI: 10.3109/14756366.2011.604852 BindingDB Entry DOI: 10.7270/Q2WH2NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237182 (Ceftizoxime) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | 8.61E+4 | -23.2 | 7.94E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Erzincan University | Assay Description CA activity was assayed according to method of Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] described previously by Innocenti e... | J Enzyme Inhib Med Chem 27: 641-5 (2012) Article DOI: 10.3109/14756366.2011.604852 BindingDB Entry DOI: 10.7270/Q2WH2NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione peroxidase 1 (Homo sapiens (Human)) | BDBM237182 (Ceftizoxime) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.21E+5 | n/a | 1.86E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Ondokuz Mayis University | Assay Description Glutathione peroxidase was assayed in a l-ml system containing 0.1 M potassium phosphate buffer, pH 7.0, 0.2 mM NADPH, 1 i.u. glutathione reductase, ... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal glutathione S-transferase 1 (Homo sapiens (Human)) | BDBM237182 (Ceftizoxime) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.65E+5 | n/a | 2.78E+5 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ondokuz Mayis University | Assay Description Enzymatic activity was determined spectrophotometrically by measuring the conjugation of CDNB with GSH. The 1 ml assay mixture contained 0.5 mM CDNB,... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||