

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor (Mus musculus-MOUSE) | BDBM50002477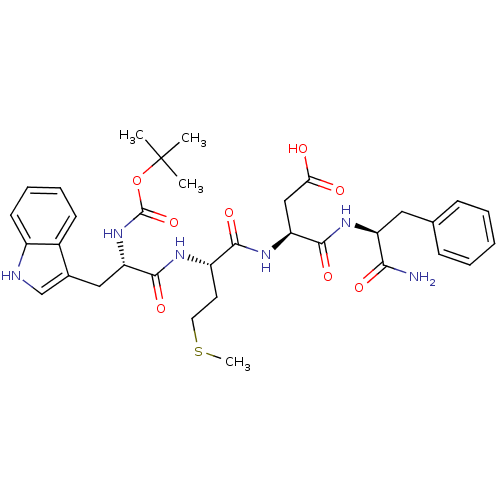 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]pentagastrin from Cholecystokinin receptor of mouse cerebral cortex | J Med Chem 30: 729-32 (1987) BindingDB Entry DOI: 10.7270/Q2KK9F0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type B receptor on guinea pig cortex. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Affinity against Cholecystokinin type A receptor on guinea pig pancreatic membranes. | J Med Chem 43: 3614-23 (2000) BindingDB Entry DOI: 10.7270/Q2RN373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||