 Found 16 hits Enz. Inhib. hit(s) with Target = '5-hydroxytryptamine receptor 4' and Ligand = 'BDBM50122872'
Found 16 hits Enz. Inhib. hit(s) with Target = '5-hydroxytryptamine receptor 4' and Ligand = 'BDBM50122872' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872
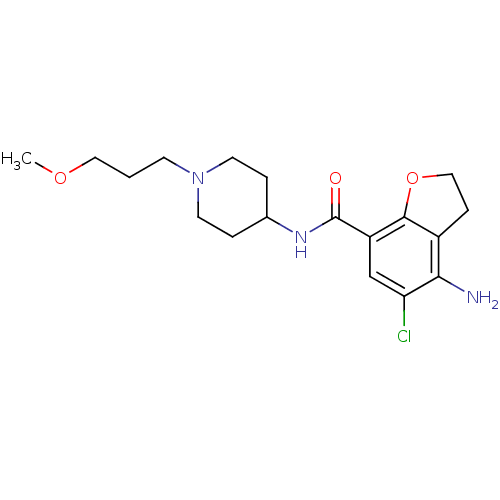
(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 423: 71-83 (2001)
Article DOI: 10.1016/s0014-2999(01)01087-1
BindingDB Entry DOI: 10.7270/Q23T9FSX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for agonist activity against 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 319-44 (2003)
BindingDB Entry DOI: 10.7270/Q2T72J6J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 423: 71-83 (2001)
Article DOI: 10.1016/s0014-2999(01)01087-1
BindingDB Entry DOI: 10.7270/Q23T9FSX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 44.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN
US Patent
| Assay Description
Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... |
US Patent US9663465 (2017)
BindingDB Entry DOI: 10.7270/Q23J3G2W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Neurochem 74: 478-89 (2000)
Article DOI: 10.1046/j.1471-4159.2000.740478.x
BindingDB Entry DOI: 10.7270/Q2DJ5D5Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Neurochem 74: 478-89 (2000)
Article DOI: 10.1046/j.1471-4159.2000.740478.x
BindingDB Entry DOI: 10.7270/Q2DJ5D5Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Neurochem 74: 478-89 (2000)
Article DOI: 10.1046/j.1471-4159.2000.740478.x
BindingDB Entry DOI: 10.7270/Q2DJ5D5Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for selectivity for 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 319-44 (2003)
BindingDB Entry DOI: 10.7270/Q2T72J6J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for selectivity for 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 319-44 (2003)
BindingDB Entry DOI: 10.7270/Q2T72J6J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for selectivity for 5-hydroxytryptamine 4 receptor |
J Med Chem 46: 319-44 (2003)
BindingDB Entry DOI: 10.7270/Q2T72J6J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Suven Life Sciences Limited
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT4E receptor (unknown origin) |
J Med Chem 61: 4993-5008 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00457
BindingDB Entry DOI: 10.7270/Q21R6T32 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Suven Life Sciences Limited
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human 5-HT4E receptor expressed in CHO cells assessed as induction of c-AMP accumulation after 4 hrs by luciferase re... |
J Med Chem 61: 4993-5008 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00457
BindingDB Entry DOI: 10.7270/Q21R6T32 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at rat 5HT4e receptor expressed in HEK293 cells assessed as cAMP level after 30 mins by HTRF assay |
Eur J Med Chem 103: 289-301 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.051
BindingDB Entry DOI: 10.7270/Q20C4ZST |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay |
Eur J Med Chem 103: 289-301 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.051
BindingDB Entry DOI: 10.7270/Q20C4ZST |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50122872

(4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...)Show InChI InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data