

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50160154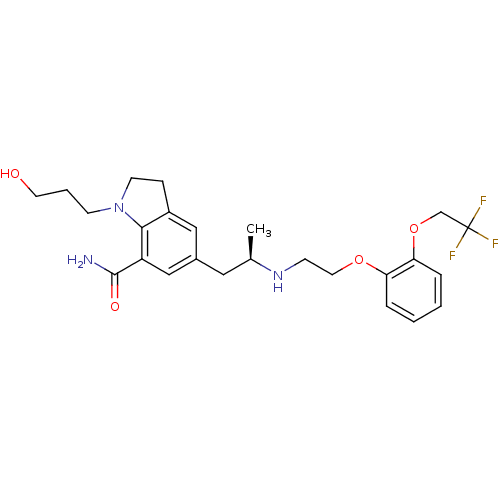 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | DrugBank Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Binding constant measured against Alpha-1A adrenergic receptor in human prostate; +++:highly active | Bioorg Med Chem Lett 15: 657-64 (2005) Article DOI: 10.1016/j.bmcl.2004.11.032 BindingDB Entry DOI: 10.7270/Q2SN08GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at alpha1A adrenoceptor in Sprague-Dawley rat urethra assessed as inhibition of norepinephrine-induced smooth muscle contraction ... | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at human full-length N-terminal SNAP-tagged alpha1A adrenoceptor expressed in HEK293 cells assessed as inhibition of agonist-indu... | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Antagonist activity at human alpha-1A adrenergic receptor transfected in HEK293 cells assessed as reduction in agonist-induced calcium mobilization a... | J Med Chem 59: 3826-39 (2016) Article DOI: 10.1021/acs.jmedchem.5b02023 BindingDB Entry DOI: 10.7270/Q22N5461 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||