

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50160154 1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluoro-ethoxy)-phenoxy]-ethylamino}-propyl)-2,3-dihydro-1H-indole-7-carboxylic acid amide::CHEMBL24778::SILODOSIN
SMILES: C[C@H](Cc1cc2CCN(CCCO)c2c(c1)C(N)=O)NCCOc1ccccc1OCC(F)(F)F
InChI Key: InChIKey=PNCPYILNMDWPEY-QGZVFWFLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50160154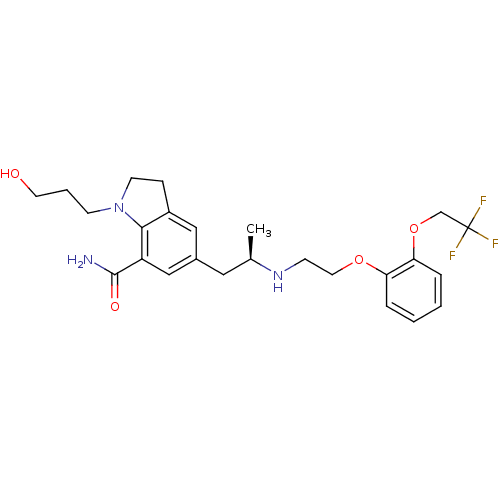 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | DrugBank Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Binding constant measured against Alpha-1A adrenergic receptor in human prostate; +++:highly active | Bioorg Med Chem Lett 15: 657-64 (2005) Article DOI: 10.1016/j.bmcl.2004.11.032 BindingDB Entry DOI: 10.7270/Q2SN08GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of rapid delayed inward rectifying potassium current (IKr) in Chinese hamster ovary (CHO) K1 cells stably expressing hERG measured using I... | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated potassium channel subunit Kv4.3 (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of transient outward potassium current (Ito) current in Chinese Hamster Ovary (CHO) K1 cells expressing human Kv4.3 measured using IonWork... | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of fast sodium current (INa) in Chinese Hamster Ovary (CHO) K1 cells transfected with human Nav1.5 measured using IonWorks Quattro automat... | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated potassium channel beta subunit Mink/subunit Kv7.1 (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of slow delayed inward rectifying potassium current (Iks) in Chinese Hamster Ovary (CHO) cells expressing hKvLQT1/hminK measured using Ion... | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of rapid delayed inward rectifying potassium current (IKr) measured using manual patch clamp assay | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | DrugBank PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Antagonist activity at alpha-1D adrenergic receptor (unknown origin) transfected in HEK293 cells assessed as reduction in agonist-induced calcium mob... | J Med Chem 59: 3826-39 (2016) BindingDB Entry DOI: 10.7270/Q22N5461 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | DrugBank PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Antagonist activity at human alpha-1A adrenergic receptor transfected in HEK293 cells assessed as reduction in agonist-induced calcium mobilization a... | J Med Chem 59: 3826-39 (2016) BindingDB Entry DOI: 10.7270/Q22N5461 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic alpha1B (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 541 | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Antagonist activity at human alpha-1B adrenergic receptor transfected in HEK293 cells assessed as reduction in agonist-induced calcium mobilization a... | J Med Chem 59: 3826-39 (2016) BindingDB Entry DOI: 10.7270/Q22N5461 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at alpha1A adrenoceptor in Sprague-Dawley rat urethra assessed as inhibition of norepinephrine-induced smooth muscle contraction ... | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at human full-length N-terminal SNAP-tagged alpha1D adrenoceptor expressed in HEK293 cells assessed as inhibition of agonist-indu... | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at human full-length N-terminal SNAP-tagged alpha1A adrenoceptor expressed in HEK293 cells assessed as inhibition of agonist-indu... | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic alpha1B (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at human full-length N-terminal SNAP-tagged alpha1B adrenoceptor expressed in HEK293 cells assessed as inhibition of agonist-indu... | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha adrenergic receptor 1A and 1B (Rattus norvegicus (rat)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at alpha1B adrenoceptor in Sprague-Dawley rat aorta assessed as inhibition of norepinephrine-induced smooth muscle contraction af... | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium channel (Type L) (Homo sapiens (Human)) | BDBM50160154 (1-(3-Hydroxy-propyl)-5-((R)-2-{2-[2-(2,2,2-trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of slow delayed inward rectifying potassium current (Iks) in Chinese Hamster Ovary (CHO) cells expressing hKvLQT1/hminK measured using Ion... | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||