

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50113678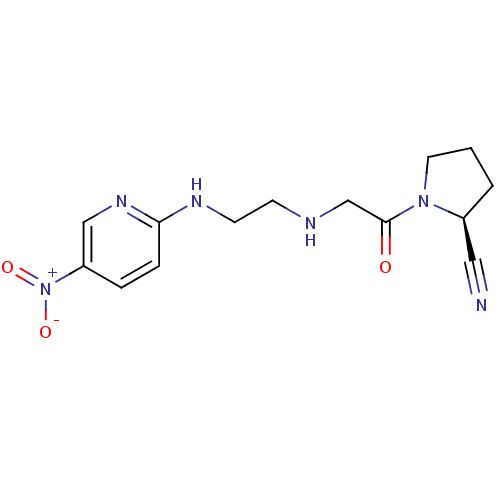 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) extracted from Caco-2 cells | J Med Chem 45: 2362-5 (2002) BindingDB Entry DOI: 10.7270/Q2930SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50113678 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50113678 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) extracted from human plasma | J Med Chem 45: 2362-5 (2002) BindingDB Entry DOI: 10.7270/Q2930SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50113678 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP IV) obtained from human plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50113678 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) extracted from rat plasma | J Med Chem 45: 2362-5 (2002) BindingDB Entry DOI: 10.7270/Q2930SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50113678 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP IV) obtained from rat plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Bos taurus) | BDBM50113678 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dipeptidyl-peptidase II (DPP-II) extracted from bovine kidney homogenate | J Med Chem 45: 2362-5 (2002) BindingDB Entry DOI: 10.7270/Q2930SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||