 Found 8 hits Enz. Inhib. hit(s) with Target = 'Procathepsin L' and Ligand = 'BDBM50033762'
Found 8 hits Enz. Inhib. hit(s) with Target = 'Procathepsin L' and Ligand = 'BDBM50033762' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Mus musculus) | BDBM50033762
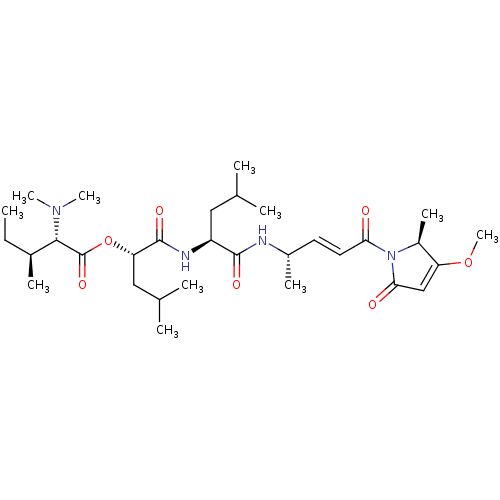
(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Cathepsin L using Z-Phe-Arg-aminomethylcoumarin as substrate preincubated for 30 mins followed by substrate addition |
J Nat Prod 77: 92-9 (2014)
Article DOI: 10.1021/np400727r
BindingDB Entry DOI: 10.7270/Q2Z89GC6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Cathepsin L using Z-Phe-Arg-aminomethylcoumarin as substrate |
J Nat Prod 77: 92-9 (2014)
Article DOI: 10.1021/np400727r
BindingDB Entry DOI: 10.7270/Q2Z89GC6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant human cathepsin L using Z-Phe-Arg-aminomethylcoumarin as substrate |
J Nat Prod 77: 92-9 (2014)
Article DOI: 10.1021/np400727r
BindingDB Entry DOI: 10.7270/Q2Z89GC6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data